Modulation of monosodium urate crystal-induced responses in neutrophils by the myeloid inhibitory C-type lectin-like receptor: potential therapeutic implications
- PMID: 23837669
- PMCID: PMC3978892
- DOI: 10.1186/ar4250
Modulation of monosodium urate crystal-induced responses in neutrophils by the myeloid inhibitory C-type lectin-like receptor: potential therapeutic implications
Abstract
Introduction: Monosodium urate crystals (MSU), the etiological agent of gout, are one of the most potent proinflammatory stimuli for neutrophils. The modulation of MSU-induced neutrophil activation by inhibitory receptors remains poorly characterized. The expression of the myeloid inhibitory C-type lectin-like receptor (MICL) in neutrophils is downregulated by several proinflammatory stimuli, suggestive of a role for this receptor in neutrophil function. We thus investigated the potential role of MICL in MSU-induced neutrophil activation.
Methods: The expression of MICL was monitored in human neutrophils by flow cytometry and Western blot analysis after stimulation with MSU. Protein tyrosine phosphorylation was also assessed by Western blot analysis and the production of IL-1 and IL-8 by enzyme-linked immunosorbent assay. Changes in the concentration of cytoplasmic free calcium were monitored with the Fura-2-acetoxymethyl ester calcium indicator. MICL expression was modulated with an anti-MICL antibody in neutrophils and siRNA in the PLB-985 neutrophil-like cell line.
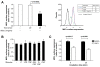
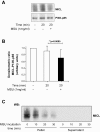
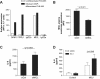
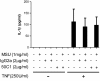
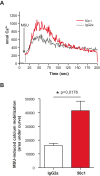
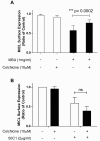
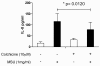
Results: MSU induced the downregulation of MICL expression in neutrophils. A diminution in the expression of MICL induced by antibody cross-linking or siRNA enhanced the MSU-dependent increase in cytoplasmic calcium levels, protein tyrosine phosphorylation and IL-8 but not IL-1 production. Pretreatment of neutrophils with colchicine inhibited the MSU-induced downregulation of MICL expression.
Conclusions: Our findings strongly suggest that MICL acts as an inhibitory receptor in human neutrophils since the downregulation of MICL expression enhances MSU-induced neutrophil activation. Since MSU downregulates the expression of MICL, MICL may play a pathogenic role in gout by enhancing neutrophil effector functions. In support of this notion, colchicine counteracts the MSU-induced loss of MICL expression. Our findings thus also provide further insight into the potential molecular mechanisms behind the anti-inflammatory properties of this drug.
Figures

Comment in
-
How to regulate neutrophils in gout.Arthritis Res Ther. 2013;15(5):118. doi: 10.1186/ar4316. Arthritis Res Ther. 2013. PMID: 24063679 Free PMC article.
Similar articles
-
The Role of Inhibitory Receptors in Monosodium Urate Crystal-Induced Inflammation.Front Immunol. 2018 Aug 20;9:1883. doi: 10.3389/fimmu.2018.01883. eCollection 2018. Front Immunol. 2018. PMID: 30177932 Free PMC article. Review.
-
How to regulate neutrophils in gout.Arthritis Res Ther. 2013;15(5):118. doi: 10.1186/ar4316. Arthritis Res Ther. 2013. PMID: 24063679 Free PMC article.
-
Melanocortin peptides inhibit urate crystal-induced activation of phagocytic cells.Arthritis Res Ther. 2009;11(5):R151. doi: 10.1186/ar2827. Epub 2009 Oct 8. Arthritis Res Ther. 2009. PMID: 19814819 Free PMC article.
-
Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation.Inflamm Res. 2017 Mar;66(3):227-237. doi: 10.1007/s00011-016-1008-0. Epub 2016 Nov 16. Inflamm Res. 2017. PMID: 27853847 Free PMC article.
-
Crystal-induced neutrophil activation.Immunol Cell Biol. 2010 Jan;88(1):32-40. doi: 10.1038/icb.2009.98. Epub 2009 Dec 1. Immunol Cell Biol. 2010. PMID: 19949421 Review.
Cited by
-
The Role of Inhibitory Receptors in Monosodium Urate Crystal-Induced Inflammation.Front Immunol. 2018 Aug 20;9:1883. doi: 10.3389/fimmu.2018.01883. eCollection 2018. Front Immunol. 2018. PMID: 30177932 Free PMC article. Review.
-
Therapeutic potential of colchicine in cardiovascular medicine: a pharmacological review.Acta Pharmacol Sin. 2022 Sep;43(9):2173-2190. doi: 10.1038/s41401-021-00835-w. Epub 2022 Jan 19. Acta Pharmacol Sin. 2022. PMID: 35046517 Free PMC article. Review.
-
Emerging insights into the role of IL-1 inhibitors and colchicine for inflammation control in type 2 diabetes.Diabetol Metab Syndr. 2024 Jun 25;16(1):140. doi: 10.1186/s13098-024-01369-x. Diabetol Metab Syndr. 2024. Retraction in: Diabetol Metab Syndr. 2025 Jan 20;17(1):21. doi: 10.1186/s13098-025-01596-w. PMID: 38918878 Free PMC article. Retracted. Review.
-
Expression of the myeloid inhibitory receptor CLEC12A correlates with disease activity and cytokines in early rheumatoid arthritis.Sci Rep. 2021 May 27;11(1):11248. doi: 10.1038/s41598-021-90631-7. Sci Rep. 2021. PMID: 34045571 Free PMC article.
-
Targeting Immunophenotypic Markers on Leukemic Stem Cells: How Lessons from Current Approaches and Advances in the Leukemia Stem Cell (LSC) Model Can Inform Better Strategies for Treating Acute Myeloid Leukemia (AML).Cold Spring Harb Perspect Med. 2020 Jan 2;10(1):a036251. doi: 10.1101/cshperspect.a036251. Cold Spring Harb Perspect Med. 2020. PMID: 31451539 Free PMC article. Review.
References
-
- Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;15(Suppl 5):S9–S12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

