Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms
- PMID: 23837896
- PMCID: PMC3843975
- DOI: 10.1111/jcmm.12100
Human endometrial stem cells confer enhanced myocardial salvage and regeneration by paracrine mechanisms
Abstract
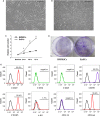
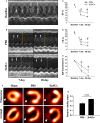
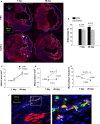
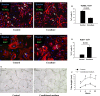
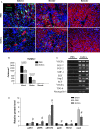
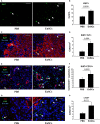
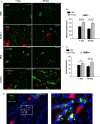
Human endometrial stem cells (EnSCs) have the potential to be 'off the shelf' clinical reagents for the treatment of heart failure. Here, using an immunocompetent rat model of myocardial infarction (MI), we provide evidence that the functional benefits of EnSC transplantation are principally and possibly exclusively through a paracrine effect. Human EnSCs were delivered by intramyocardial injection into rats 30 min. after coronary ligation. EnSC therapy significantly preserved viable myocardium in the infarct zone and improved cardiac function at 28 days. Despite increased viable myocardium and vascular density, there was scant evidence of differentiation of EnSCs into any cardiovascular cell type. Cultured human EnSCs expressed a distinctive profile of cytokines that enhanced the survival, proliferation and function of endothelial cells in vitro. When injected into the peri-infarct zone, human EnSCs activated AKT, ERK1/2 and STAT3 and inhibited the p38 signalling pathway. EnSC therapy decreased apoptosis and promoted cell proliferation and c-kit+ cell recruitment in vivo. Myocardial protection and enhanced post-infarction regeneration by EnSCs is mediated primarily by paracrine effects conferred by secreted cytokines that activate survival pathways and recruit endogenous progenitor stem cells. Menstrual blood provides a potentially limitless source of biologically competent 'off the shelf' EnSCs for allogeneic myocardial regenerative medicine.
Keywords: angiogenesis; apoptosis; endometrial stem cells; myocardial infarction; paracrine; regeneration.
© 2013 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

