A collaborative approach to developing an electronic health record phenotyping algorithm for drug-induced liver injury
- PMID: 23837993
- PMCID: PMC3861914
- DOI: 10.1136/amiajnl-2013-001930
A collaborative approach to developing an electronic health record phenotyping algorithm for drug-induced liver injury
Abstract
Objective: To describe a collaborative approach for developing an electronic health record (EHR) phenotyping algorithm for drug-induced liver injury (DILI).
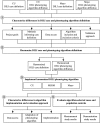
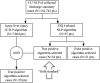
Methods: We analyzed types and causes of differences in DILI case definitions provided by two institutions-Columbia University and Mayo Clinic; harmonized two EHR phenotyping algorithms; and assessed the performance, measured by sensitivity, specificity, positive predictive value, and negative predictive value, of the resulting algorithm at three institutions except that sensitivity was measured only at Columbia University.
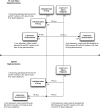
Results: Although these sites had the same case definition, their phenotyping methods differed by selection of liver injury diagnoses, inclusion of drugs cited in DILI cases, laboratory tests assessed, laboratory thresholds for liver injury, exclusion criteria, and approaches to validating phenotypes. We reached consensus on a DILI phenotyping algorithm and implemented it at three institutions. The algorithm was adapted locally to account for differences in populations and data access. Implementations collectively yielded 117 algorithm-selected cases and 23 confirmed true positive cases.
Discussion: Phenotyping for rare conditions benefits significantly from pooling data across institutions. Despite the heterogeneity of EHRs and varied algorithm implementations, we demonstrated the portability of this algorithm across three institutions. The performance of this algorithm for identifying DILI was comparable with other computerized approaches to identify adverse drug events.
Conclusions: Phenotyping algorithms developed for rare and complex conditions are likely to require adaptive implementation at multiple institutions. Better approaches are also needed to share algorithms. Early agreement on goals, data sources, and validation methods may improve the portability of the algorithms.
Keywords: Drug-induced liver injury; Electronic health records; Pharmacovigilance; Phenotyping; Rare diseases.
Figures

References
-
- Bjornsson E, Jerlstad P, Bergqvist A, et al. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005;40:1095–101 - PubMed
-
- Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl 2004;10:1018–23 - PubMed
-
- Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002;137:947–54 - PubMed
-
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med 2006;354:731–9 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

