Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia--implications for immunotherapy
- PMID: 23838315
- PMCID: PMC3778051
- DOI: 10.1158/1078-0432.CCR-13-0955
Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia--implications for immunotherapy
Abstract
Purpose: Although modern cure rates for childhood acute lymphoblastic leukemia (ALL) exceed 80%, the outlook remains poor in patients with high-risk disease and those who relapse, especially when allogeneic hematopoietic stem cell transplantation is not feasible. Strategies to improve outcome and prevent relapse are therefore required. Immunotherapy with antigen-specific T cells can have antileukemic activity without the toxicities seen with intensive chemotherapy, and therefore represents an attractive strategy to improve the outcome of high-risk patients with ALL. We explored the feasibility of generating tumor antigen-specific T cells ex vivo from the peripheral blood of 50 patients with ALL [26 National Cancer Institute (NCI) high-risk and 24 standard-risk] receiving maintenance therapy.
Experimental design: Peripheral blood mononuclear cells were stimulated with autologous dendritic cells pulsed with complete peptide libraries of WT1, Survivin, MAGE-A3, and PRAME, antigens frequently expressed on ALL blasts.
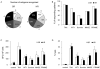
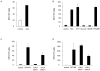
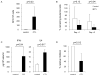
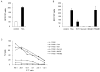
Results: T-cell lines were successfully expanded from all patients, despite low lymphocyte counts and irrespective of NCI risk group. Antigen-specificity was observed in more than 50% of patients after the initial stimulation and increased to more than 90% after three stimulations as assessed in IFN-γ-enzyme-linked immunospot (ELISpot) and (51)Cr-release assays. Moreover, tumor-specific responses were observed by reduction of autologous leukemia blasts in short- and long-term coculture experiments.
Conclusion: This study supports the use of immunotherapy with adoptively transferred autologous tumor antigen-specific T cells to prevent relapse and improve the prognosis of patients with high-risk ALL.
©2013 AACR.
Figures



References
-
- Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2008 Nov;14(11):1245–52. - PubMed
-
- Eapen M, Zhang MJ, Devidas M, Raetz E, Barredo JC, Ritchey AK, et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with acute lymphoblastic leukemia in a second remission after an isolated central nervous system relapse: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Leukemia. 2008 Feb;22(2):281–6. - PubMed
-
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995 Sep 1;86(5):2041–50. - PubMed
-
- Porter DL, Orloff GJ, Antin JH. Donor mononuclear cell infusions as therapy for B-cell lymphoproliferative disorder following allogeneic bone marrow transplant. Transplant Sci. 1994 Sep;4(1):12–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

