VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro
- PMID: 23838428
- PMCID: PMC3763768
- DOI: 10.1016/j.ajpath.2013.05.031
VEGF-A165b is an endogenous neuroprotective splice isoform of vascular endothelial growth factor A in vivo and in vitro
Abstract
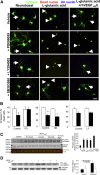
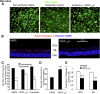
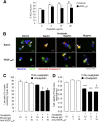
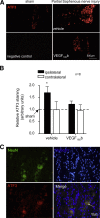
Vascular endothelial growth factor (VEGF) A is generated as two isoform families by alternative RNA splicing, represented by VEGF-A165a and VEGF-A165b. These isoforms have opposing actions on vascular permeability, angiogenesis, and vasodilatation. The proangiogenic VEGF-A165a isoform is neuroprotective in hippocampal, dorsal root ganglia, and retinal neurons, but its propermeability, vasodilatatory, and angiogenic properties limit its therapeutic usefulness. In contrast, a neuroprotective effect of endogenous VEGF-A165b on neurons would be advantageous for neurodegenerative pathologies. Endogenous expression of human and rat VEGF-A165b was detected in hippocampal and cortical neurons. VEGF-A165b formed a significant proportion of total VEGF-A in rat brain. Recombinant human VEGF-A165b exerted neuroprotective effects in response to multiple insults, including glutamatergic excitotoxicity in hippocampal neurons, chemotherapy-induced cytotoxicity of dorsal root ganglion neurons, and retinal ganglion cells (RGCs) in rat retinal ischemia-reperfusion injury in vivo. Neuroprotection was dependent on VEGFR2 and MEK1/2 activation but not on p38 or phosphatidylinositol 3-kinase activation. Recombinant human VEGF-A165b is a neuroprotective agent that effectively protects both peripheral and central neurons in vivo and in vitro through VEGFR2, MEK1/2, and inhibition of caspase-3 induction. VEGF-A165b may be therapeutically useful for pathologies that involve neuronal damage, including hippocampal neurodegeneration, glaucoma diabetic retinopathy, and peripheral neuropathy. The endogenous nature of VEGF-A165b expression suggests that non-isoform-specific inhibition of VEGF-A (for antiangiogenic reasons) may be damaging to retinal and sensory neurons.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Kovacs Z., Ikezaki K., Samoto K., Inamura T., Fukui M. VEGF and flt: expression time kinetics in rat brain infarct. Stroke. 1996;27:1865–1872. - PubMed
-
- Bates D.O., Cui T.G., Doughty J.M., Winkler M., Sugiono M., Shields J.D., Peat D., Gillatt D., Harper S.J. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. - PubMed
-
- Woolard J., Wang W.Y., Bevan H.S., Qiu Y., Morbidelli L., Pritchard-Jones R.O., Cui T.G., Sugiono M., Waine E., Perrin R., Foster R., Digby-Bell J., Shields J.D., Whittles C.E., Mushens R.E., Gillatt D.A., Ziche M., Harper S.J., Bates D.O. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

