Phosphorylation: a fundamental regulator of steroid receptor action
- PMID: 23838532
- PMCID: PMC3783573
- DOI: 10.1016/j.tem.2013.05.008
Phosphorylation: a fundamental regulator of steroid receptor action
Abstract
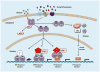
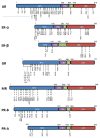
Steroid hormone receptors (SHRs) are hormone-activated transcription factors involved in numerous cellular functions and in health and disease. Their activities depend on the cellular level of the receptor, the presence of coregulator proteins, and the cell signaling pathways that are active in the cell. SHRs and their coregulators are phosphorylated on multiple sites by a wide variety of kinases. Each site may contribute to multiple functions and the net effect of an individual phosphorylation depends on the activating kinase. Here we discuss functions of known SHR phosphorylation sites, kinase regulation, evidence of translational relevance, and crosstalk between SHRs and cell signaling pathways. Understanding how cell signaling pathways regulate SHRs might yield novel therapeutic targets for multiple human diseases.
Keywords: cell signaling; coactivator; phosphorylation; steroid receptor.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
-
- Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. - PubMed
-
- Lu NZ, et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. - PubMed
-
- Walters KA, et al. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update. 2010;16:543–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

