Developmental stage- and concentration-specific sodium nitroprusside application results in nitrate reductase regulation and the modification of nitrate metabolism in leaves of Medicago truncatula plants
- PMID: 23838961
- PMCID: PMC4011814
- DOI: 10.4161/psb.25479
Developmental stage- and concentration-specific sodium nitroprusside application results in nitrate reductase regulation and the modification of nitrate metabolism in leaves of Medicago truncatula plants
Abstract
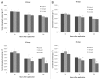
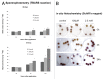
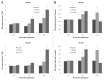
Nitric oxide (NO) is a bioactive molecule involved in numerous biological events that has been reported to display both pro-oxidant and antioxidant properties in plants. Several reports exist which demonstrate the protective action of sodium nitroprusside (SNP), a widely used NO donor, which acts as a signal molecule in plants responsible for the expression regulation of many antioxidant enzymes. This study attempts to provide a novel insight into the effect of application of low (100 μΜ) and high (2.5 mM) concentrations of SNP on the nitrosative status and nitrate metabolism of mature (40 d) and senescing (65 d) Medicago truncatula plants. Higher concentrations of SNP resulted in increased NO content, cellular damage levels and reactive oxygen species (ROS) concentration, further induced in older tissues. Senescing M. truncatula plants demonstrated greater sensitivity to SNP-induced oxidative and nitrosative damage, suggesting a developmental stage-dependent suppression in the plant's capacity to cope with free oxygen and nitrogen radicals. In addition, measurements of the activity of nitrate reductase (NR), a key enzyme involved in the generation of NO in plants, indicated a differential regulation in a dose and time-dependent manner. Furthermore, expression levels of NO-responsive genes (NR, nitrate/nitrite transporters) involved in nitrogen assimilation and NO production revealed significant induction of NR and nitrate transporter during long-term 2.5 mM SNP application in mature plants and overall gene suppression in senescing plants, supporting the differential nitrosative response of M. truncatula plants treated with different concentrations of SNP.
Keywords: Medicago truncatula; antioxidants; hydrogen peroxide; nitrate reductase; nitric oxide; nitrosative stress; oxidative stress; sodium nitroprusside.
Figures



References
-
- Corpas FJ, Chaki M, Fernández-Ocaña A, Valderrama R, Palma JM, Carreras A, et al. Metabolism of reactive nitrogen species in pea plants under abiotic stress conditions. Plant Cell Physiol. 2008;49:1711–1722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
