FKBPs and the Akt/mTOR pathway
- PMID: 23839048
- PMCID: PMC3841315
- DOI: 10.4161/cc.25508
FKBPs and the Akt/mTOR pathway
Abstract
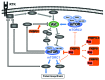
FK506-binding proteins (FKBP) belong to the immunophilin family and are best known for their ability to enable the immunosuppressive properties of FK506 and rapamycin. For rapamycin, this is achieved by inducing inhibitory ternary complexes with the kinase mTOR. The essential accessory protein for this gain-of-function was thought to be FKBP12. We recently showed that this view might be too restricted, since larger FK506-binding proteins can functionally substitute for FKBP12 in mammalian cells. Recent studies have also shown that FK506-binding proteins can modulate Akt-mTOR signaling in the absence of rapamycin. Here we discuss the role of FK506-binding proteins for the mechanism of rapamycin as well as their intrinsic actions on the Akt/mTOR pathway.
Keywords: Akt; FK506 binding protein; FKBP; PKB; mTOR; rapamycin.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
