Rotavirus specific plasma secretory immunoglobulin in children with acute gastroenteritis and children vaccinated with an attenuated human rotavirus vaccine
- PMID: 23839157
- PMCID: PMC3981851
- DOI: 10.4161/hv.25610
Rotavirus specific plasma secretory immunoglobulin in children with acute gastroenteritis and children vaccinated with an attenuated human rotavirus vaccine
Abstract
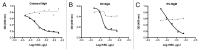
Rotavirus (RV)-specific secretory immunoglobulin (RV-SIg) has been previously detected in serum of naturally RV infected children and shown to reflect the intestinal Ig immune response. Total plasma SIgA and plasma RV-SIg were evaluated by ELISA in children with gastroenteritis due or not due to RV infection and in 50 children vaccinated with the attenuated RIX4414 human RV vaccine and 62 placebo recipients. RV-SIg was only detected in children with evidence of previous RV infection or with acute RV gastroenteritis. Vaccinees had higher RV-SIg titers than placebo recipients and RV-SIg titers increased after the second vaccine dose. RV-SIg measured after the second dose correlated with protection when vaccinees and placebo recipients were analyzed jointly. RV-SIg may serve as a valuable correlate of protection for RV vaccines.
Keywords: correlate of protection; rotavirus; secretory immunoglobulin; vaccine.
Figures



References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
