Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair
- PMID: 23839576
- PMCID: PMC3757945
- DOI: 10.1038/nrm3619
Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair
Abstract
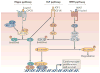
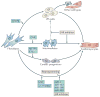
As the adult mammalian heart has limited potential for regeneration and repair, the loss of cardiomyocytes during injury and disease can result in heart failure and death. The cellular processes and regulatory mechanisms involved in heart growth and development can be exploited to repair the injured adult heart through 'reawakening' pathways that are active during embryogenesis. Heart function has been restored in rodents by reprogramming non-myocytes into cardiomyocytes, by expressing transcription factors (GATA4, HAND2, myocyte-specific enhancer factor 2C (MEF2C) and T-box 5 (TBX5)) and microRNAs (miR-1, miR-133, miR-208 and miR-499) that control cardiomyocyte identity. Stimulating cardiomyocyte dedifferentiation and proliferation by activating mitotic signalling pathways involved in embryonic heart growth represents a complementary approach for heart regeneration and repair. Recent advances in understanding the mechanistic basis of heart development offer exciting opportunities for effective therapies for heart failure.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

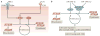

References
-
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. - PubMed
-
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
-
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. - PubMed
-
- Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nature Rev Cardiol. 2010;7:204–215. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

