The Golgi puppet master: COG complex at center stage of membrane trafficking interactions
- PMID: 23839779
- PMCID: PMC3748202
- DOI: 10.1007/s00418-013-1117-6
The Golgi puppet master: COG complex at center stage of membrane trafficking interactions
Abstract
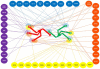
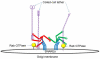
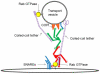
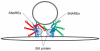
The central organelle within the secretory pathway is the Golgi apparatus, a collection of flattened membranes organized into stacks. The cisternal maturation model of intra-Golgi transport depicts Golgi cisternae that mature from cis to medial to trans by receiving resident proteins, such as glycosylation enzymes via retrograde vesicle-mediated recycling. The conserved oligomeric Golgi (COG) complex, a multi-subunit tethering complex of the complexes associated with tethering containing helical rods family, organizes vesicle targeting during intra-Golgi retrograde transport. The COG complex, both physically and functionally, interacts with all classes of molecules maintaining intra-Golgi trafficking, namely SNAREs, SNARE-interacting proteins, Rabs, coiled-coil tethers, vesicular coats, and molecular motors. In this report, we will review the current state of the COG interactome and analyze possible scenarios for the molecular mechanism of the COG orchestrated vesicle targeting, which plays a central role in maintaining glycosylation homeostasis in all eukaryotic cells.
Figures
References
-
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. - PubMed
-
- Boxem M, Maliga Z, Klitgord N, Li N, Lemmens I, Mana M, de Lichtervelde L, Mul JD, van de Peut D, Devos M, Simonis N, Yildirim MA, Cokol M, Kao HL, de Smet AS, Wang H, Schlaitz AL, Hao T, Milstein S, Fan C, Tipsword M, Drew K, Galli M, Rhrissorrakrai K, Drechsel D, Koller D, Roth FP, Iakoucheva LM, Dunker AK, Bonneau R, Gunsalus KC, Hill DE, Piano F, Tavernier J, van den Heuvel S, Hyman AA, Vidal M. A protein domain-based interactome network for C. elegans early embryogenesis. Cell. 2008;134(3):534–545. doi:10.1016/j.cell.2008.07.009. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

