Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21
- PMID: 23839791
- PMCID: PMC3830593
- DOI: 10.1002/oby.20559
Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21
Retraction in
-
Retraction.Obesity (Silver Spring). 2023 Apr;31(4):1170. doi: 10.1002/oby.23735. Epub 2023 Feb 27. Obesity (Silver Spring). 2023. PMID: 36849874 Free PMC article.
Abstract
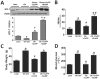
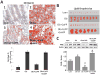
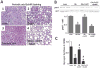
Objective: Obese leptin deficient (ob/ob) mice are a model of adiposity that displays increased levels of fat, glucose, and liver lipids. Our hypothesis is that HO-1 overexpression ameliorates fatty liver development.
Methods: Obese mice were administered cobalt protoporphyrin (CoPP) and stannic mesoporphyrin (SnMP) for 6 weeks. Heme, HO-1, HO activity, PGC1α, FGF21, glycogen content, and lipogenesis were assessed.
Results: CoPP administration increased hepatic HO-1 protein levels and HO activity, decreased hepatic heme, body weight gain, glucose levels, and resulted in decreased steatosis. Increased levels of HO-1 produced a decrease in lipid droplet size, Fatty acid synthase (FAS) levels involving recruitment of FGF21, PPARα, and Glut 1. These beneficial effects were reversed by inhibition of HO activity.
Conclusion: Increased levels of HO-1 and HO activity reduced the levels of obesity by reducing hepatic heme and lipid accumulation. These changes were manifested by decreases in cellular heme, increases in FGF21, glycogen content, and fatty liver. The beneficial effect of HO-1 induction results from an increase in PPARα and FGF21 levels and a decrease in PGC1α, levels they were reversed by SnMP. Low levels of HO-1 and HO activity are responsible for fatty liver.
Copyright © 2013 The Obesity Society.
Conflict of interest statement
Figures
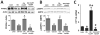

References
-
- Krahenbuhl L, Lang C, Ludes S, Seiler C, Schafer M, Zimmermann A, et al. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 2003;23:101–109. - PubMed
-
- Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol. 2009;104:861–867. - PubMed
-
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

