Isolating contributions from intersegmental transfer to DNA searching by alkyladenine DNA glycosylase
- PMID: 23839988
- PMCID: PMC3750153
- DOI: 10.1074/jbc.M113.477018
Isolating contributions from intersegmental transfer to DNA searching by alkyladenine DNA glycosylase
Abstract
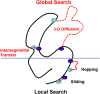
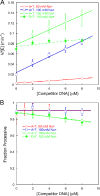
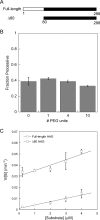
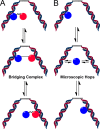
Large genomes pose a challenge to DNA repair pathways because rare sites of damage must be efficiently located from among a vast excess of undamaged sites. Human alkyladenine DNA glycosylase (AAG) employs nonspecific DNA binding interactions and facilitated diffusion to conduct a highly redundant search of adjacent sites. This ensures that every site is searched, but could be a detriment if the protein is trapped in a local segment of DNA. Intersegmental transfer between DNA segments that are transiently in close proximity provides an elegant solution that balances global and local searching processes. It has been difficult to detect intersegmental transfer experimentally; therefore, we developed biochemical assays that allowed us to observe and measure the rates of intersegmental transfer by AAG. AAG has a flexible amino terminus that tunes its affinity for nonspecific DNA, but we find that it is not required for intersegmental transfer. As AAG has only a single DNA binding site, this argues against the bridging model for intersegmental transfer. The rates of intersegmental transfer are strongly dependent on the salt concentration, supporting a jumping mechanism that involves microscopic dissociation and capture by a proximal DNA site. As many DNA-binding proteins have only a single binding site, jumping may be a common mechanism for intersegmental transfer.
Keywords: Base Excision Repair; DNA Glycosylase; DNA Repair; Enzyme Kinetics; Enzyme Mechanisms; Facilitated Diffusion; Intersegmental Transfer; Mutagenesis Mechanisms.
Figures


References
-
- Berg O. G., Winter R. B., von Hippel P. H. (1981) Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry 20, 6929–6948 - PubMed
-
- Iwahara J., Clore G. M. (2006) Direct observation of enhanced translocation of a homeodomain between DNA cognate sites by NMR exchange spectroscopy. J. Am. Chem. Soc. 128, 404–405 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

