Fifty years of DNA "breathing": Reflections on old and new approaches
- PMID: 23840028
- PMCID: PMC4313903
- DOI: 10.1002/bip.22347
Fifty years of DNA "breathing": Reflections on old and new approaches
Abstract
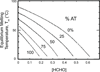
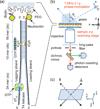
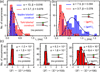
The coding sequences for genes, and much other regulatory information involved in genome expression, are located 'inside' the DNA duplex. Thus the "macromolecular machines" that read-out this information from the base sequence of the DNA must somehow access the DNA "interior." Double-stranded (ds) DNA is a highly structured and cooperatively stabilized system at physiological temperatures, but is also only marginally stable and undergoes a cooperative "melting phase transition" at temperatures not far above physiological. Furthermore, due to its length and heterogeneous sequence, with AT-rich segments being less stable than GC-rich segments, the DNA genome 'melts' in a multistate fashion. Therefore the DNA genome must also manifest thermally driven structural ("breathing") fluctuations at physiological temperatures that should reflect the heterogeneity of the dsDNA stability near the melting temperature. Thus many of the breathing fluctuations of dsDNA are likely also to be sequence dependent, and could well contain information that should be "readable" and useable by regulatory proteins and protein complexes in site-specific binding reactions involving dsDNA "opening." Our laboratory has been involved in studying the breathing fluctuations of duplex DNA for about 50 years. In this "Reflections" article we present a relatively chronological overview of these studies, starting with the use of simple chemical probes (such as hydrogen exchange, formaldehyde, and simple DNA "melting" proteins) to examine the local stability of the dsDNA structure, and culminating in sophisticated spectroscopic approaches that can be used to monitor the breathing-dependent interactions of regulatory complexes with their duplex DNA targets in "real time."
Keywords: DNA base analogue spectroscopy; DNA replication; formaldehyde probing; hydrogen exchange; protein-nucleic acid interactions; single molecule DNA-protein interactions studies; two-dimensional fluorescence spectroscopy.
Copyright © 2013 Wiley Periodicals, Inc.
Figures












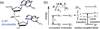


References
-
- Watson JD, Crick FH. Nature. 1953;171:737–738. - PubMed
-
- Bessman MJ, Lehman IR, Simms ES, Kornberg A. J Biol Chem. 1958;233:171–177. - PubMed
-
- Lehman IR, Bessman MJ, Simms ES, Kornberg A. J Biol Chem. 1958;233:163–170. - PubMed
-
- Benson EE, Linderstrom-Lang K. Biochimica et biophysica acta. 1959;32:579–581. - PubMed
-
- Linderstrom-Lang K. Biochimica et biophysica acta. 1955;18:308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

