Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery
- PMID: 23840043
- PMCID: PMC3733506
- DOI: 10.1093/infdis/jit236
Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery
Abstract
Background: DNA vaccines have been very poorly immunogenic in humans but have been an effective priming modality in prime-boost regimens. Methods to increase the immunogenicity of DNA vaccines are needed.
Methods: HIV Vaccine Trials Network (HVTN) studies 070 and 080 were multicenter, randomized, clinical trials. The human immunodeficiency virus type 1 (HIV-1) PENNVAX®-B DNA vaccine (PV) is a mixture of 3 expression plasmids encoding HIV-1 Clade B Env, Gag, and Pol. The interleukin 12 (IL-12) DNA plasmid expresses human IL-12 proteins p35 and p40. Study subjects were healthy HIV-1-uninfected adults 18-50 years old. Four intramuscular vaccinations were given in HVTN 070, and 3 intramuscular vaccinations were followed by electroporation in HVTN 080. Cellular immune responses were measured by intracellular cytokine staining after stimulation with HIV-1 peptide pools.
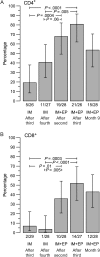
Results: Vaccination was safe and well tolerated. Administration of PV plus IL-12 with electroporation had a significant dose-sparing effect and provided immunogenicity superior to that observed in the trial without electroporation, despite fewer vaccinations. A total of 71.4% of individuals vaccinated with PV plus IL-12 plasmid with electroporation developed either a CD4(+) or CD8(+) T-cell response after the second vaccination, and 88.9% developed a CD4(+) or CD8(+) T-cell response after the third vaccination.
Conclusions: Use of electroporation after PV administration provided superior immunogenicity than delivery without electroporation. This study illustrates the power of combined DNA approaches to generate impressive immune responses in humans.
Keywords: electroporation; plasmid cytokine adjuvant; vaccination.
Figures




References
-
- Hokey DA, Weiner DB. DNA vaccines for HIV: challenges and opportunities. Springer Semin Immunopathol. 2006;28:267–79. - PubMed
-
- Catanzaro AT, Roederer M, Koup RA, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–92. - PubMed
-
- MacGregor RR, Boyer JD, Ugen KE, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1TR000445/TR/NCATS NIH HHS/United States
- HHSN272200800063C/AI/NIAID NIH HHS/United States
- UM1 AI069421/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UM1 AI068618/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
- UM1AI069421/AI/NIAID NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- P01 AI071739/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068614/AI/NIAID NIH HHS/United States
- P01-AI071739/AI/NIAID NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI068635/AI/NIAID NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

