Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites
- PMID: 23840061
- PMCID: PMC3725082
- DOI: 10.1073/pnas.1222276110
Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites
Abstract
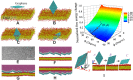
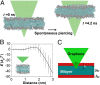
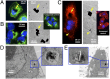
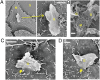
Understanding and controlling the interaction of graphene-based materials with cell membranes is key to the development of graphene-enabled biomedical technologies and to the management of graphene health and safety issues. Very little is known about the fundamental behavior of cell membranes exposed to ultrathin 2D synthetic materials. Here we investigate the interactions of graphene and few-layer graphene (FLG) microsheets with three cell types and with model lipid bilayers by combining coarse-grained molecular dynamics (MD), all-atom MD, analytical modeling, confocal fluorescence imaging, and electron microscopic imaging. The imaging experiments show edge-first uptake and complete internalization for a range of FLG samples of 0.5- to 10-μm lateral dimension. In contrast, the simulations show large energy barriers relative to kBT for membrane penetration by model graphene or FLG microsheets of similar size. More detailed simulations resolve this paradox by showing that entry is initiated at corners or asperities that are abundant along the irregular edges of fabricated graphene materials. Local piercing by these sharp protrusions initiates membrane propagation along the extended graphene edge and thus avoids the high energy barrier calculated in simple idealized MD simulations. We propose that this mechanism allows cellular uptake of even large multilayer sheets of micrometer-scale lateral dimension, which is consistent with our multimodal bioimaging results for primary human keratinocytes, human lung epithelial cells, and murine macrophages.
Keywords: corner penetration; edge cutting; graphene-cell interaction; lipid membrane; molecular dynamics simulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Zhang Y, Tan Y-W, Stormer HL, Kim P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature. 2005;438(7065):201–204. - PubMed
-
- Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008;321(5887):385–388. - PubMed
-
- Balandin AA, et al. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008;8(3):902–907. - PubMed
-
- Shih C-J, et al. Bi- and trilayer graphene solutions. Nat Nanotechnol. 2011;6(7):439–445. - PubMed
-
- Huang X, et al. Graphene-based materials: Synthesis, characterization, properties, and applications. Small. 2011;7(14):1876–1902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

