Role of activin A in carbon tetrachloride-induced acute liver injury
- PMID: 23840118
- PMCID: PMC3699031
- DOI: 10.3748/wjg.v19.i24.3802
Role of activin A in carbon tetrachloride-induced acute liver injury
Abstract
Aim: To investigate the expression and role of activin A in a mouse model of acute chemical liver injury.
Methods: Acute liver injury in C57BL/6 male mice was induced by intraperitoneal injection with carbon tetrachloride (CCl4) (0.5 mL/kg, body weight) dissolved in olive oil (1:19 v/v). Mice were sacrificed 1, 3, 5 and 7 d after the treatment. The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in serum were examined and pathological changes of liver observed by hematoxylin and eosin staining to evaluate the liver injury. Activin A protein levels in serum and hepatic tissue homogenate of mice were detected by enzyme-linked immunosorbent assay, and the expression pattern of activin A protein in livers of mice was examined by immunohistochemistry. Activin type IIA receptor (ActRIIA) and Smad3 expressions in the liver were analyzed by real-time quantitative reverse transcription-polymerase chain reaction. In order to further investigate the role of activin A, we also utilized activin A blocking experiment by anti-activin A antibody (500 μg/kg, body weight) injection into mouse tail vein.
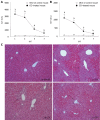
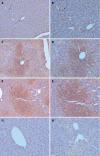
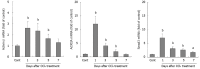
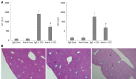
Results: In CCl4-treated mice, serum ALT and AST levels were significantly increased, compared with that in control mice (P < 0.01). Furthermore, the serious necrosis was observed around hepatic portal areas in CCl4-treated mice. Simultaneously, activin A levels in serum and hepatic tissue homogenate of mice treated with CCl4 for 1, 3 and 5 d increased significantly, compared with that in control mice (P < 0.01). Activin A protein expression in hepatocytes not within the necrotic area was also upregulated in mice following CCl4 treatment. Not only activin A, but also ActRIIA and activin signaling molecule Smad3 mRNA expressions in injury liver induced by CCl4 were significantly higher than that in control liver. In addition, levels of serum ALT and AST in CCl4-treated mice were significantly decreased by injection of anti-activin A antibody to block endogenous activin A action, compared with that in CCl4-treated mice by injection of immunoglobulin G instead of anti-activin A antibody (P < 0.01), and the severity of liver injury was also reduced remarkably.
Conclusion: These data show that activin A is involved in CCl4-induced acute liver injury. Blocking activin A actions may be a therapeutic approach for acute liver injury.
Keywords: Activin A; Carbon tetrachloride; Immunohistochemistry; Liver injury.
Figures

References
-
- Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. - PubMed
-
- Chen YG, Lui HM, Lin SL, Lee JM, Ying SY. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 2002;227:75–87. - PubMed
-
- Phillips DJ, de Kretser DM, Hedger MP. Activin and related proteins in inflammation: not just interested bystanders. Cytokine Growth Factor Rev. 2009;20:153–164. - PubMed
-
- Wang SY, Tai GX, Zhang PY, Mu DP, Zhang XJ, Liu ZH. Inhibitory effect of activin A on activation of lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Cytokine. 2008;42:85–91. - PubMed
-
- Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol. 2006;177:6787–6794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

