Severe irinotecan-induced toxicity in a patient with UGT1A1 28 and UGT1A1 6 polymorphisms
- PMID: 23840132
- PMCID: PMC3699040
- DOI: 10.3748/wjg.v19.i24.3899
Severe irinotecan-induced toxicity in a patient with UGT1A1 28 and UGT1A1 6 polymorphisms
Abstract
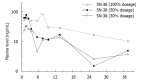
Many studies have demonstrated the impact of UGT1A1 on toxicity of irinotecan. In particular, patients bearing UGT1A1 28 (TA 7/7) have a higher risk of severe neutropenia and diarrhea. Based on this, prescribers of irinotecan are advised that patients with UGT1A1 28 (TA 7/7) should start with a reduced dose of irinotecan, although a particular dose is not specified. Research in Asian countries has shown a lower incidence of UGT1A1 28 (TA 7/7), while UGT1A1 6 (A/A) is more often found and is associated with severe irinotecan-related neutropenia. We report here a case of a metastatic colorectal cancer patient who is heterozygous for the UGT1A1 28 polymorphism (TA 6/7) as well as the UGT1A1 6 polymorphism (G/A). The patient was treated with FOLFIRI for 9 cycles and underwent two irinotecan dose reductions according to pharmacokinetic data regarding exposure to the active metabolite, SN-38. Simultaneous heterozygous UGT1A1 28 and UGT1A1 6 polymorphisms may produce higher exposure to SN-38 and a higher risk of adverse effects related to irinotecan. Additional studies will be necessary to determine the optimal starting dose of irinotecan for patients with both UGT1A1 28 and UGT1A1 6 polymorphisms.
Keywords: Irinotecan; Polymorphism; Toxicity; UGT1A1*28; UGT1A1*6.
Figures
Similar articles
-
Relationship between UGT1A1*6/*28 polymorphisms and severe toxicities in Chinese patients with pancreatic or biliary tract cancer treated with irinotecan-containing regimens.Drug Des Devel Ther. 2015 Jul 17;9:3677-83. doi: 10.2147/DDDT.S86750. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26229432 Free PMC article.
-
[Relationship between UGT1A1 gene polymorphisms and irinotecan-induced severe adverse events].Zhonghua Zhong Liu Za Zhi. 2018 Aug 23;40(8):594-599. doi: 10.3760/cma.j.issn.0253-3766.2018.08.006. Zhonghua Zhong Liu Za Zhi. 2018. PMID: 30139029 Chinese.
-
Pharmacokinetics, safety, and efficacy of FOLFIRI plus bevacizumab in Japanese colorectal cancer patients with UGT1A1 gene polymorphisms.J Clin Pharmacol. 2014 May;54(5):495-502. doi: 10.1002/jcph.246. Epub 2014 Jan 2. J Clin Pharmacol. 2014. PMID: 24382596 Clinical Trial.
-
UGT1A1 polymorphisms with irinotecan-induced toxicities and treatment outcome in Asians with Lung Cancer: a meta-analysis.Cancer Chemother Pharmacol. 2017 Jun;79(6):1109-1117. doi: 10.1007/s00280-017-3306-9. Epub 2017 May 13. Cancer Chemother Pharmacol. 2017. PMID: 28502040 Review.
-
UGT1A1*28 and other UGT1A polymorphisms as determinants of irinotecan toxicity.J Chemother. 2008 Apr;20(2):158-65. doi: 10.1179/joc.2008.20.2.158. J Chemother. 2008. PMID: 18467239 Review.
Cited by
-
Toward an understanding of the pathophysiology of clear cell carcinoma of the ovary (Review).Oncol Lett. 2013 Nov;6(5):1163-1173. doi: 10.3892/ol.2013.1550. Epub 2013 Aug 28. Oncol Lett. 2013. PMID: 24179489 Free PMC article.
-
The regioselective glucuronidation of morphine by dimerized human UGT2B7, 1A1, 1A9 and their allelic variants.Acta Pharmacol Sin. 2017 Aug;38(8):1184-1194. doi: 10.1038/aps.2016.157. Epub 2017 May 29. Acta Pharmacol Sin. 2017. PMID: 28552915 Free PMC article.
-
Development of Pyrosequencing Method for Detection of UGT1A1 Polymorphisms in Thai Colorectal Cancers.J Clin Lab Anal. 2016 Jan;30(1):84-9. doi: 10.1002/jcla.21820. Epub 2014 Dec 26. J Clin Lab Anal. 2016. PMID: 25545261 Free PMC article.
-
Clinical Study on Prevention of Irinotecan-Induced Delayed-Onset Diarrhea by Hot Ironing with Moxa Salt Packet on Tianshu and Shangjuxu.Emerg Med Int. 2022 Jul 21;2022:6587884. doi: 10.1155/2022/6587884. eCollection 2022. Emerg Med Int. 2022. Retraction in: Emerg Med Int. 2024 Jan 24;2024:9814198. doi: 10.1155/2024/9814198. PMID: 35912389 Free PMC article. Retracted.
-
Using Literature-Based Discovery to Explain Adverse Drug Effects.J Med Syst. 2016 Aug;40(8):185. doi: 10.1007/s10916-016-0544-z. Epub 2016 Jun 18. J Med Syst. 2016. PMID: 27318993
References
-
- Di Paolo A, Bocci G, Polillo M, Del Re M, Di Desidero T, Lastella M, Danesi R. Pharmacokinetic and pharmacogenetic predictive markers of irinotecan activity and toxicity. Curr Drug Metab. 2011;12:932–943. - PubMed
-
- Cecchin E, Innocenti F, D’Andrea M, Corona G, De Mattia E, Biason P, Buonadonna A, Toffoli G. Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol. 2009;27:2457–2465. - PubMed
-
- Strassburg CP, Kalthoff S, Ehmer U. Variability and function of family 1 uridine-5’-diphosphate glucuronosyltransferases (UGT1A) Crit Rev Clin Lab Sci. 2008;45:485–530. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

