Arginase as a mediator of diabetic retinopathy
- PMID: 23840196
- PMCID: PMC3699717
- DOI: 10.3389/fimmu.2013.00173
Arginase as a mediator of diabetic retinopathy
Abstract
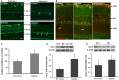
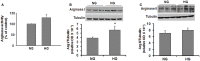
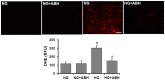
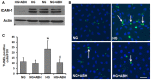
We have shown previously that diabetes causes increases in retinal arginase activity that are associated with impairment of endothelial cell (EC)-dependent vasodilation and increased formation of the peroxynitrite biomarker nitrotyrosine. Arginase blockade normalizes vasodilation responses and reduces nitrotyrosine formation, suggesting that overactive arginase contributes to diabetic retinopathy by reducing NO and increasing oxidative stress. We tested this hypothesis by studies in streptozotocin-induced diabetic mice and high glucose (HG) treated retinal ECs. Our results show that arginase activity is increased in both diabetic retinas and HG-treated retinal ECs as compared with the controls. Western blot shows that both arginase isoforms are present in retinal vessels and ECs and arginase I is increased in the diabetic vessels and HG-treated retinal ECs. Nitrate/nitrite levels are significantly increased in diabetic retinas, indicating an increase in total NO products. However, levels of nitrite, an indicator of bioavailable NO, are reduced by diabetes. Imaging analysis of NO formation in retinal sections confirmed decreases in NO formation in diabetic retinas. The decrease in NO is accompanied by increased [Formula: see text] formation and increased leukocyte attachment in retinal vessels. Studies in knockout mice show that arginase gene deletion enhances NO formation, reduces [Formula: see text] and prevents leukostasis in the diabetic retinas. HG treatment of retinal ECs also reduces NO release, increases oxidative stress, increases ICAM-1, and induces EC death. Arginase inhibitor treatment reverses these effects. In conclusion, diabetes- and HG-induced signs of retinal vascular activation and injury are associated with increased arginase activity and expression, decreased bioavailable NO, and increased [Formula: see text] formation. Blockade of the arginase pathway prevents these alterations, suggesting a primary role of arginase in the pathophysiological process.
Keywords: arginase; diabetes; diabetic retinopathy; high glucose; nitric oxide; oxidative stress.
Figures




References
-
- NEI The Prevalence of Diabetic Retinopathy Among Adults in the United States (2008). Available from: http://www.nei.nih.gov/eyedata/pbd3.asp
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

