HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic
- PMID: 23840329
- PMCID: PMC3686785
- DOI: 10.1371/journal.pone.0065453
HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic
Abstract
Background: Pulmonary fibrosis may result from abnormal alveolar wound repair after injury. Hepatocyte growth factor (HGF) improves alveolar epithelial wound repair in the lung. Stem cells were shown to play a major role in lung injury, repair and fibrosis. We studied the presence, origin and antifibrotic properties of HGF-expressing stem cells in usual interstitial pneumonia.
Methods: Immunohistochemistry was performed in lung tissue sections and primary alveolar epithelial cells obtained from patients with usual interstitial pneumonia (UIP, n = 7). Bone marrow derived stromal cells (BMSC) from adult male rats were transfected with HGF, instilled intratracheally into bleomycin injured rat lungs and analyzed 7 and 14 days later.
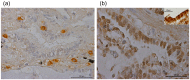
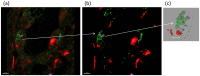
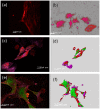
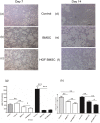
Results: In UIP, HGF was expressed in specific cells mainly located in fibrotic areas close to the hyperplastic alveolar epithelium. HGF-positive cells showed strong co-staining for the mesenchymal stem cell markers CD44, CD29, CD105 and CD90, indicating stem cell origin. HGF-positive cells also co-stained for CXCR4 (HGF+/CXCR4+) indicating that they originate from the bone marrow. The stem cell characteristics were confirmed in HGF secreting cells isolated from UIP lung biopsies. In vivo experiments showed that HGF-expressing BMSC attenuated bleomycin induced pulmonary fibrosis in the rat, indicating a beneficial role of bone marrow derived, HGF secreting stem cells in lung fibrosis.
Conclusions: HGF-positive stem cells are present in human fibrotic lung tissue (UIP) and originate from the bone marrow. Since HGF-transfected BMSC reduce bleomycin induced lung fibrosis in the bleomycin lung injury and fibrosis model, we assume that HGF-expressing, bone-marrow derived stem cells in UIP have antifibrotic properties.
Conflict of interest statement
Figures




Similar articles
-
The secretome of induced pluripotent stem cells reduces lung fibrosis in part by hepatocyte growth factor.Stem Cell Res Ther. 2014 Nov 10;5(6):123. doi: 10.1186/scrt513. Stem Cell Res Ther. 2014. PMID: 25384638 Free PMC article.
-
Gene transfer of hepatocyte growth factor by electroporation reduces bleomycin-induced lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2007 Feb;292(2):L529-36. doi: 10.1152/ajplung.00082.2006. Epub 2006 Oct 20. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17056705
-
Targeted gene transfer of hepatocyte growth factor to alveolar type II epithelial cells reduces lung fibrosis in rats.Hum Gene Ther. 2013 Jan;24(1):105-16. doi: 10.1089/hum.2012.098. Hum Gene Ther. 2013. PMID: 23134111
-
Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration.Stem Cells Transl Med. 2021 Jul;10(7):1021-1032. doi: 10.1002/sctm.20-0526. Epub 2021 Feb 24. Stem Cells Transl Med. 2021. PMID: 33624948 Free PMC article. Review.
-
Usual interstitial pneumonia: a review of the pathogenesis and discussion of elastin fibres, type II pneumocytes and proposed roles in the pathogenesis.Pathology. 2022 Aug;54(5):517-525. doi: 10.1016/j.pathol.2022.05.002. Epub 2022 Jun 29. Pathology. 2022. PMID: 35778287 Review.
Cited by
-
Targeting mesenchymal stem cell therapy for severe pneumonia patients.World J Stem Cells. 2021 Feb 26;13(2):139-154. doi: 10.4252/wjsc.v13.i2.139. World J Stem Cells. 2021. PMID: 33708343 Free PMC article. Review.
-
HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis.Int J Mol Sci. 2021 Feb 11;22(4):1790. doi: 10.3390/ijms22041790. Int J Mol Sci. 2021. PMID: 33670243 Free PMC article.
-
Suppression of NLRP3 inflammasome by ivermectin ameliorates bleomycin-induced pulmonary fibrosis.J Zhejiang Univ Sci B. 2023 Jul 1;24(8):723-733. doi: 10.1631/jzus.B2200385. J Zhejiang Univ Sci B. 2023. PMID: 37551558 Free PMC article.
-
Mesenchymal stem cell therapy in pulmonary fibrosis: a meta-analysis of preclinical studies.Stem Cell Res Ther. 2021 Aug 18;12(1):461. doi: 10.1186/s13287-021-02496-2. Stem Cell Res Ther. 2021. PMID: 34407861 Free PMC article.
-
Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease.Nat Rev Drug Discov. 2017 Oct 30;16(11):810. doi: 10.1038/nrd.2017.225. Nat Rev Drug Discov. 2017. PMID: 29081515 Free PMC article.
References
-
- Nathan SD, Shlobin OA, Weir N, Ahmad S, Kaldjob JM, et al. (2011) Long-term Course and Prognosis of Idiopathic Pulmonary Fibrosis in the New Millennium. Chest 140: 221–229. - PubMed
-
- Wuyts WA, Agostini C, Antoniou K, Bouros D, Chambers R, et al.. (2012) The pathogenesis of pulmonary fibrosis: a moving target. European Respiratory Journal. - PubMed
-
- Geiser T (2003 ) Mechanisms of alveolar epithelial repair in acute lung injury – a translational approach. Swiss Med Wkly 133: 586–590. - PubMed
-
- Selman M, King TE, Pardo A (2001) Idiopathic Pulmonary Fibrosis: Prevailing and Evolving Hypotheses about Its Pathogenesis and Implications for Therapy. Annals of Internal Medicine 134: 136–151. - PubMed
-
- Leslie KO (2011) Idiopathic Pulmonary Fibrosis May Be a Disease of Recurrent, Tractional Injury to the Periphery of the Aging Lung: A Unifying Hypothesis Regarding Etiology and Pathogenesis. Arch Pathol Lab Med. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

