Development of an HIV-1 Microbicide Based on Caulobacter crescentus: Blocking Infection by High-Density Display of Virus Entry Inhibitors
- PMID: 23840383
- PMCID: PMC3686833
- DOI: 10.1371/journal.pone.0065965
Development of an HIV-1 Microbicide Based on Caulobacter crescentus: Blocking Infection by High-Density Display of Virus Entry Inhibitors
Abstract
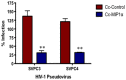
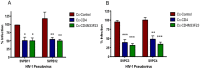
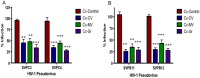
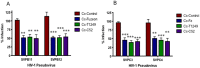
The HIV/AIDS pandemic remains an enormous global health concern. Despite effective prevention options, 2.6 million new infections occur annually, with women in developing countries accounting for more than half of these infections. New prevention strategies that can be used by women are urgently needed. Topical microbicides specific for HIV-1 represent a promising prevention strategy. Conceptually, using harmless bacteria to display peptides or proteins capable of blocking entry provides an inexpensive approach to microbicide development. To avoid the potential pitfalls of engineering commensal bacteria, our strategy is to genetically display infection inhibitors on a non-native bacterium and rely on topical application of stabilized bacteria before potential virus exposure. Due to the high density cell-surface display capabilities and the inherent low toxicity of the bacterium, the S-layer mediated protein display capabilities of the non-pathogenic bacterium Caulobacter crescentus has been exploited for this approach. We have demonstrated that C. crescentus displaying MIP1α or CD4 interfered with the virus entry pathway and provided significant protection from HIV-1 pseudovirus representing clade B in a standard single cycle infection assay. Here we have expanded our C. crescentus based microbicide approach with additional and diverse classes of natural and synthetic inhibitors of the HIV-1 entry pathway. All display constructs provided variable but significant protection from HIV-1 infection; some with protection as high as 70%. Further, we describe protection from infection with additional viral clades. These findings indicate the significant potential for engineering C. crescentus to be an effective and readily adaptable HIV-1 microbicide platform.
Conflict of interest statement
Figures

References
-
- UNAIDS (2011) UNAIDS Data Table 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

