Proteomic View of Interactions of Shiga Toxin-Producing Escherichia coli with the Intestinal Environment in Gnotobiotic Piglets
- PMID: 23840478
- PMCID: PMC3686733
- DOI: 10.1371/journal.pone.0066462
Proteomic View of Interactions of Shiga Toxin-Producing Escherichia coli with the Intestinal Environment in Gnotobiotic Piglets
Abstract
Background: Shiga toxin (Stx)-producing Escherichia coli cause severe intestinal infections involving colonization of epithelial Peyer's patches and formation of attachment/effacement (A/E) lesions. These lesions trigger leukocyte infiltration followed by inflammation and intestinal hemorrhage. Systems biology, which explores the crosstalk of Stx-producing Escherichia coli with the in vivo host environment, may elucidate novel molecular pathogenesis aspects.
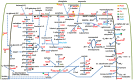
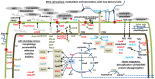
Methodology/principal findings: Enterohemorrhagic E. coli strain 86-24 produces Shiga toxin-2 and belongs to the serotype O157:H7. Bacterial cells were scrapped from stationary phase cultures (the in vitro condition) and used to infect gnotobiotic piglets via intestinal lavage. Bacterial cells isolated from the piglets' guts constituted the in vivo condition. Cell lysates were subjected to quantitative 2D gel and shotgun proteomic analyses, revealing metabolic shifts towards anaerobic energy generation, changes in carbon utilization, phosphate and ammonia starvation, and high activity of a glutamate decarboxylase acid resistance system in vivo. Increased abundance of pyridine nucleotide transhydrogenase (PntA and PntB) suggested in vivo shortage of intracellular NADPH. Abundance changes of proteins implicated in lipopolysaccharide biosynthesis (LpxC, ArnA, the predicted acyltransferase L7029) and outer membrane (OM) assembly (LptD, MlaA, MlaC) suggested bacterial cell surface modulation in response to activated host defenses. Indeed, there was evidence for interactions of innate immunity-associated proteins secreted into the intestines (GP340, REG3-γ, resistin, lithostathine, and trefoil factor 3) with the bacterial cell envelope.
Significance: Proteomic analysis afforded insights into system-wide adaptations of strain 86-24 to a hostile intestinal milieu, including responses to limited nutrients and cofactor supplies, intracellular acidification, and reactive nitrogen and oxygen species-mediated stress. Protein and lipopolysaccharide compositions of the OM were altered. Enhanced expression of type III secretion system effectors correlated with a metabolic shift back to a more aerobic milieu in vivo. Apparent pathogen pattern recognition molecules from piglet intestinal secretions adhered strongly to the bacterial cell surface.
Conflict of interest statement
Figures

Similar articles
-
Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets.Infect Immun. 1987 Dec;55(12):3117-25. doi: 10.1128/iai.55.12.3117-3125.1987. Infect Immun. 1987. PMID: 3316033 Free PMC article.
-
Characterizing the Escherichia coli O157:H7 proteome including protein associations with higher order assemblies.PLoS One. 2011;6(11):e26554. doi: 10.1371/journal.pone.0026554. Epub 2011 Nov 7. PLoS One. 2011. PMID: 22087229 Free PMC article.
-
Expansion of Shiga Toxin-Producing Escherichia coli by Use of Bovine Antibiotic Growth Promoters.Emerg Infect Dis. 2016 May;22(5):802-9. doi: 10.3201/eid2205.151584. Emerg Infect Dis. 2016. PMID: 27088186 Free PMC article.
-
A Toxic Environment: a Growing Understanding of How Microbial Communities Affect Escherichia coli O157:H7 Shiga Toxin Expression.Appl Environ Microbiol. 2020 Nov 24;86(24):e00509-20. doi: 10.1128/AEM.00509-20. Print 2020 Nov 24. Appl Environ Microbiol. 2020. PMID: 32358004 Free PMC article. Review.
-
A secretome view of colonisation factors in Shiga toxin-encoding Escherichia coli (STEC): from enterohaemorrhagic E. coli (EHEC) to related enteropathotypes.FEMS Microbiol Lett. 2016 Aug;363(16):fnw179. doi: 10.1093/femsle/fnw179. Epub 2016 Jul 26. FEMS Microbiol Lett. 2016. PMID: 27465489 Review.
Cited by
-
Comparative Transcriptomics of Shiga Toxin-Producing and Commensal Escherichia coli and Cytokine Responses in Colonic Epithelial Cell Culture Infections.Front Cell Infect Microbiol. 2020 Oct 26;10:575630. doi: 10.3389/fcimb.2020.575630. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194815 Free PMC article.
-
Pathogenic potential assessment of the Shiga toxin-producing Escherichia coli by a source attribution-considered machine learning model.Proc Natl Acad Sci U S A. 2021 May 18;118(20):e2018877118. doi: 10.1073/pnas.2018877118. Proc Natl Acad Sci U S A. 2021. PMID: 33986113 Free PMC article.
-
PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation.PLoS One. 2014 Apr 7;9(4):e94285. doi: 10.1371/journal.pone.0094285. eCollection 2014. PLoS One. 2014. PMID: 24710330 Free PMC article.
-
Opening a Novel Biosynthetic Pathway to Dihydroxyacetone and Glycerol in Escherichia coli Mutants through Expression of a Gene Variant (fsaAA129S) for Fructose 6-Phosphate Aldolase.Int J Mol Sci. 2020 Dec 17;21(24):9625. doi: 10.3390/ijms21249625. Int J Mol Sci. 2020. PMID: 33348713 Free PMC article.
-
Proteomic Analyses of Intracellular Salmonella enterica Serovar Typhimurium Reveal Extensive Bacterial Adaptations to Infected Host Epithelial Cells.Infect Immun. 2015 Jul;83(7):2897-906. doi: 10.1128/IAI.02882-14. Epub 2015 May 4. Infect Immun. 2015. PMID: 25939512 Free PMC article.
References
-
- Perna NT, Plunkett G, 3rd, Burland V, Mau B, Glasner JD, et al (2001) Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409: 529–533. - PubMed
-
- Griffin PM, Ostroff SM, Tauxe RV, Greene KD, Wells JG, et al. (1988) Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann Intern Med 109: 705–712. - PubMed
-
- Donohue-Rolfe A, Kondova I, Oswald S, Hutto D, Tzipori S (2000) Escherichia coli O157:H7 strains that express Shiga toxin (Stx) 2 alone are more neurotropic for gnotobiotic piglets than are isotypes producing only Stx1 or both Stx1 and Stx2. J Infect Dis 181: 1825–1829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

