Niacin Reduces Atherosclerosis Development in APOE*3Leiden.CETP Mice Mainly by Reducing NonHDL-Cholesterol
- PMID: 23840481
- PMCID: PMC3686722
- DOI: 10.1371/journal.pone.0066467
Niacin Reduces Atherosclerosis Development in APOE*3Leiden.CETP Mice Mainly by Reducing NonHDL-Cholesterol
Abstract
Objective: Niacin potently lowers triglycerides, mildly decreases LDL-cholesterol, and largely increases HDL-cholesterol. Despite evidence for an atheroprotective effect of niacin from previous small clinical studies, the large outcome trials, AIM-HIGH and HPS2-THRIVE did not reveal additional beneficial effects of niacin (alone or in combination with laropiprant) on top of statin treatment. We aimed to address this apparent discrepancy by investigating the effects of niacin without and with simvastatin on atherosclerosis development and determine the underlying mechanisms, in APOE*3Leiden.CETP mice, a model for familial dysbetalipoproteinemia (FD).
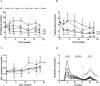
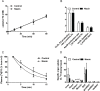
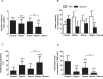
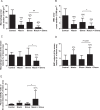
Approach and results: Mice were fed a western-type diet containing cholesterol without or with niacin (120 mg/kg/day), simvastatin (36 mg/kg/day) or their combination for 18 weeks. Similarly as in FD patients, niacin reduced total cholesterol by -39% and triglycerides by -50%, (both P<0.001). Simvastatin and the combination reduced total cholesterol (-30%; -55%, P<0.001) where the combination revealed a greater reduction compared to simvastatin (-36%, P<0.001). Niacin decreased total cholesterol and triglycerides primarily by increasing VLDL clearance. Niacin increased HDL-cholesterol (+28%, P<0.01) and mildly increased reverse cholesterol transport. All treatments reduced monocyte adhesion to the endothelium (-46%; -47%, P<0.01; -53%, P<0.001), atherosclerotic lesion area (-78%; -49%, P<0.01; -87%, P<0.001) and severity. Compared to simvastatin, the combination increased plaque stability index [(SMC+collagen)/macrophages] (3-fold, P<0.01). Niacin and the combination reduced T cells in the aortic root (-71%, P<0.01; -81%, P<0.001). Lesion area was strongly predicted by nonHDL-cholesterol (R(2) = 0.69, P<0.001) and to a much lesser extent by HDL-cholesterol (R(2) = 0.20, P<0.001).
Conclusion: Niacin decreases atherosclerosis development mainly by reducing nonHDL-cholesterol with modest HDL-cholesterol-raising and additional anti-inflammatory effects. The additive effect of niacin on top of simvastatin is mostly dependent on its nonHDL-cholesterol-lowering capacities. These data suggest that clinical beneficial effects of niacin are largely dependent on its ability to lower LDL-cholesterol on top of concomitant lipid-lowering therapy.
Conflict of interest statement
Figures




References
-
- Carlson LA (2005) Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med 258: 94–114. - PubMed
-
- Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES (2005) Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol 45: 185–197. - PubMed
-
- Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, et al. (2009) Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med 361: 2113–2122. - PubMed
-
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA (2004) Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110: 3512–3517. - PubMed
-
- Taylor AJ, Lee HJ, Sullenberger LE (2006) The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 22: 2243–2250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

