Mass fingerprinting of the venom and transcriptome of venom gland of scorpion Centruroides tecomanus
- PMID: 23840487
- PMCID: PMC3688770
- DOI: 10.1371/journal.pone.0066486
Mass fingerprinting of the venom and transcriptome of venom gland of scorpion Centruroides tecomanus
Abstract
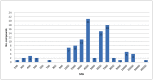
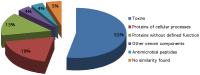
Centruroides tecomanus is a Mexican scorpion endemic of the State of Colima, that causes human fatalities. This communication describes a proteome analysis obtained from milked venom and a transcriptome analysis from a cDNA library constructed from two pairs of venom glands of this scorpion. High perfomance liquid chromatography separation of soluble venom produced 80 fractions, from which at least 104 individual components were identified by mass spectrometry analysis, showing to contain molecular masses from 259 to 44,392 Da. Most of these components are within the expected molecular masses for Na(+)- and K(+)-channel specific toxic peptides, supporting the clinical findings of intoxication, when humans are stung by this scorpion. From the cDNA library 162 clones were randomly chosen, from which 130 sequences of good quality were identified and were clustered in 28 contigs containing, each, two or more expressed sequence tags (EST) and 49 singlets with only one EST. Deduced amino acid sequence analysis from 53% of the total ESTs showed that 81% (24 sequences) are similar to known toxic peptides that affect Na(+)-channel activity, and 19% (7 unique sequences) are similar to K(+)-channel especific toxins. Out of the 31 sequences, at least 8 peptides were confirmed by direct Edman degradation, using components isolated directly from the venom. The remaining 19%, 4%, 4%, 15% and 5% of the ESTs correspond respectively to proteins involved in cellular processes, antimicrobial peptides, venom components, proteins without defined function and sequences without similarity in databases. Among the cloned genes are those similar to metalloproteinases.
Conflict of interest statement
Figures


References
-
- Chippaux J, Goyffon M (2008) Acta Tropica Epidemiology of scorpionism: A global appraisal. Acta Tropica 107: 71–79. - PubMed
-
- Fet V, Sissom WD, Lowe G, Braunwalder ME (2000) Catalog of the Scorpions of the World (1758–1998). New York: New York Entomological Society. 690 p.
-
- Monroy Velasco J, Monroy Nieto JM (1960) Alacranes venenosos de México. Revista Mexicana de Ciencias Médicas y Biológicas 1 and 2: 1–19.
-
- Hoffman CC (1931) La distribución geográfica de los “Alacranes peligrosos” en la República Mexicana. Anales del Instituto de Biología de la Universidad Nacional Autónoma de México 2: 291, 408.
-
- Chowell G, Díaz-Dueñas P, Bustos-Saldaña R, Alemán Mireles A, Fet V (2006) Epidemiological and clinical characteristics of scorpionism in Colima, Mexico (2000–2001 ). Toxicon 47: 753–758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

