Modulating the Adhesion of Haematopoietic Stem Cells with Chemokines to Enhance Their Recruitment to the Ischaemically Injured Murine Kidney
- PMID: 23840488
- PMCID: PMC3686749
- DOI: 10.1371/journal.pone.0066489
Modulating the Adhesion of Haematopoietic Stem Cells with Chemokines to Enhance Their Recruitment to the Ischaemically Injured Murine Kidney
Abstract
Introduction: Renal disease affects over 500 million people worldwide and is set to increase as treatment options are predominately supportive. Evidence suggests that exogenous haematopoietic stem cells (HSCs) can be of benefit but due to the rarity and poor homing of these cells, benefits are either minor or transitory. Mechanisms governing HSC recruitment to injured renal microcirculation are poorly understood; therefore this study determined (i) the adhesion molecules responsible for HSC recruitment to the injured kidney, (ii) if cytokine HSC pre-treatment can enhance their homing and (iii) the molecular mechanisms accountable for any enhancement.
Methods: Adherent and free-flowing HSCs were determined in an intravital murine model of renal ischaemia-reperfusion injury. Some HSCs and animals were pre-treated prior to HSC infusion with function blocking antibodies, hyaluronidase or cytokines. Changes in surface expression and clustering of HSC adhesion molecules were determined using flow cytometry and confocal microscopy. HSC adhesion to endothelial counter-ligands (VCAM-1, hyaluronan) was determined using static adhesion assays in vitro.
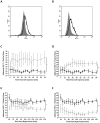
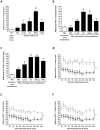
Results: CD49d, CD44, VCAM-1 and hyaluronan governed HSC adhesion to the IR-injured kidney. Both KC and SDF-1α pre-treatment strategies significantly increased HSC adhesion within injured kidney, whilst SDF-1α also increased numbers continuing to circulate. SDF-1α and KC did not increase CD49d or CD44 expression but increased HSC adhesion to VCAM-1 and hyaluronan respectively. SDF-1α increased CD49d surface clustering, as well as HSC deformability.
Conclusion: Increasing HSC adhesive capacity for its endothelial counter-ligands, potentially through surface clustering, may explain their enhanced renal retention in vivo. Furthermore, increasing HSC deformability through SDF-1α treatment could explain the prolonged systemic circulation; the HSC can therefore continue to survey the damaged tissue instead of becoming entrapped within non-injured sites. Therefore manipulating these mechanisms of HSC recruitment outlined may improve the clinical outcome of cellular therapies for kidney disease.
Conflict of interest statement
Figures




Similar articles
-
Mechanisms of adhesion and subsequent actions of a haematopoietic stem cell line, HPC-7, in the injured murine intestinal microcirculation in vivo.PLoS One. 2013;8(3):e59150. doi: 10.1371/journal.pone.0059150. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23554986 Free PMC article.
-
Haematopoietic stem cell recruitment to injured murine liver sinusoids depends on (alpha)4(beta)1 integrin/VCAM-1 interactions.Gut. 2010 Jan;59(1):79-87. doi: 10.1136/gut.2008.168054. Gut. 2010. PMID: 19828466
-
Haematopoietic stem cell migration to the ischemic damaged kidney is not altered by manipulating the SDF-1/CXCR4-axis.Nephrol Dial Transplant. 2009 Jul;24(7):2082-8. doi: 10.1093/ndt/gfp050. Epub 2009 Feb 16. Nephrol Dial Transplant. 2009. PMID: 19223274 Free PMC article.
-
Hematopoietic stem cell trafficking in liver injury.FASEB J. 2005 Aug;19(10):1225-31. doi: 10.1096/fj.04-2604rev. FASEB J. 2005. PMID: 16051689 Review.
-
Cytokines and hematopoietic stem cell mobilization.J Cell Biochem. 2006 Oct 15;99(3):690-705. doi: 10.1002/jcb.21043. J Cell Biochem. 2006. PMID: 16888804 Review.
Cited by
-
A historical review of experimental imaging of the beating heart coronary microcirculation in vivo.J Anat. 2023 Jan;242(1):3-16. doi: 10.1111/joa.13611. Epub 2021 Dec 14. J Anat. 2023. PMID: 34905637 Free PMC article. Review.
-
The Important Role of FLT3-L in Ex Vivo Expansion of Hematopoietic Stem Cells following Co-Culture with Mesenchymal Stem Cells.Cell J. 2015 Summer;17(2):201-10. doi: 10.22074/cellj.2016.3715. Epub 2015 Jul 11. Cell J. 2015. PMID: 26199899 Free PMC article.
-
Improving vasculoprotective effects of MSCs in coronary microvessels - benefits of 3D culture, sub-populations and heparin.Front Immunol. 2023 Oct 24;14:1257497. doi: 10.3389/fimmu.2023.1257497. eCollection 2023. Front Immunol. 2023. PMID: 37954606 Free PMC article.
-
Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut.Stem Cells. 2015 Sep;33(9):2785-97. doi: 10.1002/stem.2061. Epub 2015 Jun 29. Stem Cells. 2015. PMID: 26124062 Free PMC article.
-
Mesenchymal Stem Cell Deformability and Implications for Microvascular Sequestration.Ann Biomed Eng. 2018 Apr;46(4):640-654. doi: 10.1007/s10439-018-1985-y. Epub 2018 Jan 19. Ann Biomed Eng. 2018. PMID: 29352448 Free PMC article.
References
-
- Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. N Engl J Med 334: 1448–1460. - PubMed
-
- Bonventre JV, Zuk A (2004) Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485. - PubMed
-
- Agrawal M, Swartz R (2000) Acute renal failure. Am Fam Physician 61: 2077–2088. - PubMed
-
- Eltzschig HK, Collard CD (2004) Vascular ischaemia and reperfusion injury. Br Med Bull 70: 71–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

