The ArfGEF GBF-1 Is Required for ER Structure, Secretion and Endocytic Transport in C. elegans
- PMID: 23840591
- PMCID: PMC3686754
- DOI: 10.1371/journal.pone.0067076
The ArfGEF GBF-1 Is Required for ER Structure, Secretion and Endocytic Transport in C. elegans
Abstract
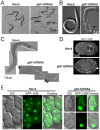
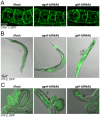
Small GTPases of the Sar/Arf family are essential to generate transport containers that mediate communication between organelles of the secretory pathway. Guanine nucleotide exchange factor (GEFs) activate the small GTPases and help their anchorage in the membrane. Thus, GEFs in a way temporally and spatially control Sar1/Arf1 GTPase activation. We investigated the role of the ArfGEF GBF-1 in C. elegans oocytes and intestinal epithelial cells. GBF-1 localizes to the cis-Golgi and is part of the t-ER-Golgi elements. GBF-1 is required for secretion and Golgi integrity. In addition, gbf-1(RNAi) causes the ER reticular structure to become dispersed, without destroying ER exit sites (ERES) because the ERES protein SEC-16 was still localized in distinct punctae at t-ER-Golgi units. Moreover, GBF-1 plays a role in receptor-mediated endocytosis in oocytes, without affecting recycling pathways. We find that both the yolk receptor RME-2 and the recycling endosome-associated RAB-11 localize similarly in control and gbf-1(RNAi) oocytes. While RAB5-positive early endosomes appear to be less prominent and the RAB-5 levels are reduced by gbf-1(RNAi) in the intestine, RAB-7-positive late endosomes were more abundant and formed aggregates and tubular structures. Our data suggest a role for GBF-1 in ER structure and endosomal traffic.
Conflict of interest statement
Figures




References
-
- Poteryaev D, Datta S, Ackema K, Zerial M, Spang A (2010) Identification of the switch in early-to-late endosome transition. Cell 141: 497–508. - PubMed
-
- Morinaga N, Tsai SC, Moss J, Vaughan M (1996) Isolation of a brefeldin A-inhibited guanine nucleotide-exchange protein for ADP ribosylation factor (ARF) 1 and ARF3 that contains a Sec7-like domain. Proceedings of the National Academy of Sciences of the United States of America 93: 12856–12860. - PMC - PubMed
-
- Peyroche A, Paris S, Jackson CL (1996) Nucleotide exchange on ARF mediated by yeast Gea1 protein. Nature 384: 479–481. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

