Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation
- PMID: 23840636
- PMCID: PMC3696042
- DOI: 10.1371/journal.pone.0067240
Staphylococcus epidermidis in orthopedic device infections: the role of bacterial internalization in human osteoblasts and biofilm formation
Abstract
Background: Staphylococcus epidermidis orthopedic device infections are caused by direct inoculation of commensal flora during surgery and remain rare, although S. epidermidis carriage is likely universal. We wondered whether S. epidermidis orthopedic device infection strains might constitute a sub-population of commensal isolates with specific virulence ability. Biofilm formation and invasion of osteoblasts by S. aureus contribute to bone and joint infection recurrence by protecting bacteria from the host-immune system and most antibiotics. We aimed to determine whether S. epidermidis orthopedic device infection isolates could be distinguished from commensal strains by their ability to invade osteoblasts and form biofilms.
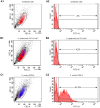
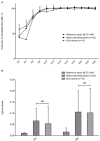
Materials and methods: Orthopedic device infection S. epidermidis strains (n = 15) were compared to nasal carriage isolates (n = 22). Osteoblast invasion was evaluated in an ex vivo infection model using MG63 osteoblastic cells co-cultured for 2 hours with bacteria. Adhesion of S. epidermidis to osteoblasts was explored by a flow cytometric approach, and internalized bacteria were quantified by plating cell lysates after selective killing of extra-cellular bacteria with gentamicin. Early and mature biofilm formations were evaluated by a crystal violet microtitration plate assay and the Biofilm Ring Test method.
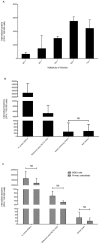
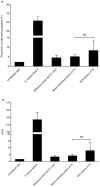
Results: No difference was observed between commensal and infective strains in their ability to invade osteoblasts (internalization rate 308+/-631 and 347+/-431 CFU/well, respectively). This low internalization rate correlated with a low ability to adhere to osteoblasts. No difference was observed for biofilm formation between the two groups.
Conclusion: Osteoblast invasion and biofilm formation levels failed to distinguish S. epidermidis orthopedic device infection strains from commensal isolates. This study provides the first assessment of the interaction between S. epidermidis strains isolated from orthopedic device infections and osteoblasts, and suggests that bone cell invasion is not a major pathophysiological mechanism in S. epidermidis orthopedic device infections, contrary to what is observed for S. aureus.
Conflict of interest statement
Figures


References
-
- Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. New Engl J Med 351: 1645–1654. - PubMed
-
- Lew DP, Waldvogel FA (2004) Osteomyelitis. Lancet 364: 369–379. - PubMed
-
- Uckay I, Pittet D, Vaudaux P, Sax H, Lew D, et al. (2009) Foreign body infections due to Staphylococcus epidermidis (2009) Ann Med. 41: 109–119. - PubMed
-
- Schierholz JM, Beuth J (2001) Implant infections: a haven for opportunistic bacteria. J Hosp Infect 49: 87–93. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

