HGF mediates the anti-inflammatory effects of PRP on injured tendons
- PMID: 23840657
- PMCID: PMC3696073
- DOI: 10.1371/journal.pone.0067303
HGF mediates the anti-inflammatory effects of PRP on injured tendons
Abstract
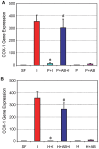
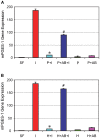
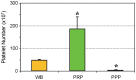
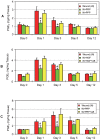
Platelet-rich plasma (PRP) containing hepatocyte growth factor (HGF) and other growth factors are widely used in orthopaedic/sports medicine to repair injured tendons. While PRP treatment is reported to decrease pain in patients with tendon injury, the mechanism of this effect is not clear. Tendon pain is often associated with tendon inflammation, and HGF is known to protect tissues from inflammatory damages. Therefore, we hypothesized that HGF in PRP causes the anti-inflammatory effects. To test this hypothesis, we performed in vitro experiments on rabbit tendon cells and in vivo experiments on a mouse Achilles tendon injury model. We found that addition of PRP or HGF decreased gene expression of COX-1, COX-2, and mPGES-1, induced by the treatment of tendon cells in vitro with IL-1β. Further, the treatment of tendon cell cultures with HGF antibodies reduced the suppressive effects of PRP or HGF on IL-1β-induced COX-1, COX-2, and mPGES-1 gene expressions. Treatment with PRP or HGF almost completely blocked the cellular production of PGE2 and the expression of COX proteins. Finally, injection of PRP or HGF into wounded mouse Achilles tendons in vivo decreased PGE2 production in the tendinous tissues. Injection of platelet-poor plasma (PPP) however, did not reduce PGE2 levels in the wounded tendons, but the injection of HGF antibody inhibited the effects of PRP and HGF. Further, injection of PRP or HGF also decreased COX-1 and COX-2 proteins. These results indicate that PRP exerts anti-inflammatory effects on injured tendons through HGF. This study provides basic scientific evidence to support the use of PRP to treat injured tendons because PRP can reduce inflammation and thereby reduce the associated pain caused by high levels of PGE2.
Conflict of interest statement
Figures








References
-
- Butler DL, Juncosa N, Dressler MR (2004) Functional efficacy of tendon repair processes. Annu Rev Biomed Eng 6: 303–329. - PubMed
-
- Ferreira SH, Nakamura M, de Abreu Castro MS (1978) The hyperalgesic effects of prostacyclin and prostaglandin E2. Prostaglandins 16: 31–37. - PubMed
-
- Kopp UC, Cicha MZ (1999) PGE2 increases substance P release from renal pelvic sensory nerves via activation of N-type calcium channels. Am J Physiol 276: R1241–1248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

