Stage-regulated GFP Expression in Trypanosoma cruzi: applications from host-parasite interactions to drug screening
- PMID: 23840703
- PMCID: PMC3688654
- DOI: 10.1371/journal.pone.0067441
Stage-regulated GFP Expression in Trypanosoma cruzi: applications from host-parasite interactions to drug screening
Abstract
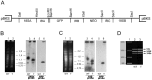
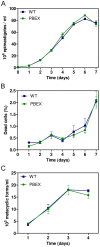
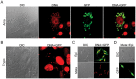
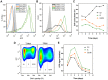
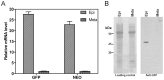
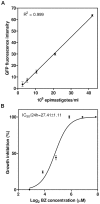
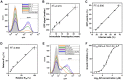
Trypanosoma cruzi is the etiological agent of Chagas disease, an illness that affects about 10 million people, mostly in South America, for which there is no effective treatment or vaccine. In this context, transgenic parasites expressing reporter genes are interesting tools for investigating parasite biology and host-parasite interactions, with a view to developing new strategies for disease prevention and treatment. We describe here the construction of a stably transfected fluorescent T. cruzi clone in which the GFP gene is integrated into the chromosome carrying the ribosomal cistron in T. cruzi Dm28c. This fluorescent T. cruzi produces detectable amounts of GFP only at replicative stages (epimastigote and amastigote), consistent with the larger amounts of GFP mRNA detected in these forms than in the non replicative trypomastigote stages. The fluorescence signal was also strongly correlated with the total number of parasites in T. cruzi cultures, providing a simple and rapid means of determining the growth inhibitory dose of anti-T.cruzi drugs in epimastigotes, by fluorometric microplate screening, and in amastigotes, by the flow cytometric quantification of T. cruzi-infected Vero cells. This fluorescent T. cruzi clone is, thus, an interesting tool for unbiased detection of the proliferating stages of the parasite, with multiple applications in the genetic analysis of T. cruzi, including analyses of host-parasite interactions, gene expression regulation and drug development.
Conflict of interest statement
Figures
References
-
- Anonymous (2010) Chagas disease (American trypanosomiasis) fact sheet (revised in June 2010). Wkly Epidemiol Rec 85: 334–336. - PubMed
-
- Coura JR, Dias JC (2009) Epidemiology, control and surveillance of Chagas disease: 100 years after its discovery. Mem Inst Oswaldo Cruz 104 Suppl 131–40. - PubMed
-
- Tyler KM, Engman DM (2001) The life cycle of Trypanosoma cruzi revisited. Int J Parasitol 31: 472–481. - PubMed
-
- De Souza W (2002) Basic cell biology of Trypanosoma cruzi . Curr Pharm Des 8: 269–285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

