MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens
- PMID: 23840749
- PMCID: PMC3695902
- DOI: 10.1371/journal.pone.0067608
MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens
Abstract
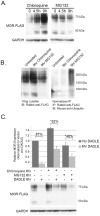
The mu-opioid receptor (MOR) is the G-protein coupled receptor primarily responsible for mediating the analgesic and rewarding properties of opioid agonist drugs such as morphine, fentanyl, and heroin. We have utilized a combination of traditional and modified membrane yeast two-hybrid screening methods to identify a cohort of novel MOR interacting proteins (MORIPs). The interaction between the MOR and a subset of MORIPs was validated in pulldown, co-immunoprecipitation, and co-localization studies using HEK293 cells stably expressing the MOR as well as rodent brain. Additionally, a subset of MORIPs was found capable of interaction with the delta and kappa opioid receptors, suggesting that they may represent general opioid receptor interacting proteins (ORIPS). Expression of several MORIPs was altered in specific mouse brain regions after chronic treatment with morphine, suggesting that these proteins may play a role in response to opioid agonist drugs. Based on the known function of these newly identified MORIPs, the interactions forming the MOR signalplex are hypothesized to be important for MOR signaling and intracellular trafficking. Understanding the molecular complexity of MOR/MORIP interactions provides a conceptual framework for defining the cellular mechanisms of MOR signaling in brain and may be critical for determining the physiological basis of opioid tolerance and addiction.
Conflict of interest statement
Figures






References
-
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, et al. (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383: 819–823. - PubMed
-
- von Zastrow M, Svingos A, Haberstock-Debic H, Evans C (2003) Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol 13: 348–353. - PubMed
-
- Koch T, Hollt V (2008) Role of receptor internalization in opioid tolerance and dependence. Pharmacol Ther 117: 199–206. - PubMed
-
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, et al. (2005) Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol 67: 280–287. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

