Epileptic discharges affect the default mode network--FMRI and intracerebral EEG evidence
- PMID: 23840805
- PMCID: PMC3695970
- DOI: 10.1371/journal.pone.0068038
Epileptic discharges affect the default mode network--FMRI and intracerebral EEG evidence
Abstract
Functional neuroimaging studies of epilepsy patients often show, at the time of epileptic activity, deactivation in default mode network (DMN) regions, which is hypothesized to reflect altered consciousness. We aimed to study the metabolic and electrophysiological correlates of these changes in the DMN regions. We studied six epilepsy patients that underwent scalp EEG-fMRI and later stereotaxic intracerebral EEG (SEEG) sampling regions of DMN (posterior cingulate cortex, Pre-cuneus, inferior parietal lobule, medial prefrontal cortex and dorsolateral frontal cortex) as well as non-DMN regions. SEEG recordings were subject to frequency analyses comparing sections with interictal epileptic discharges (IED) to IED-free baselines in the IED-generating region, DMN and non-DMN regions. EEG-fMRI and SEEG were obtained at rest. During IEDs, EEG-fMRI demonstrated deactivation in various DMN nodes in 5 of 6 patients, most frequently the pre-cuneus and inferior parietal lobule, and less frequently the other DMN nodes. SEEG analyses demonstrated decrease in gamma power (50-150 Hz), and increase in the power of lower frequencies (<30 Hz) at times of IEDs, in at least one DMN node in all patients. These changes were not apparent in the non-DMN regions. We demonstrate that, at the time of IEDs, DMN regions decrease their metabolic demand and undergo an EEG change consisting of decreased gamma and increased lower frequencies. These findings, specific to DMN regions, confirm in a pathological condition a direct relationship between DMN BOLD activity and EEG activity. They indicate that epileptic activity affects the DMN, and therefore may momentarily reduce the consciousness level and cognitive reserve.
Conflict of interest statement
Figures



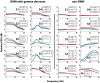
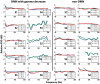
References
-
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, et al. (2007) Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett 417: 297–302. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

