Human DDX3 interacts with the HIV-1 Tat protein to facilitate viral mRNA translation
- PMID: 23840900
- PMCID: PMC3698215
- DOI: 10.1371/journal.pone.0068665
Human DDX3 interacts with the HIV-1 Tat protein to facilitate viral mRNA translation
Abstract
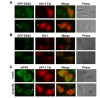
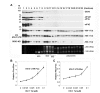
Nuclear export and translation of intron-containing viral mRNAs are required for HIV-1 gene expression and replication. In this report, we provide evidence to show that DDX3 regulates the translation of HIV-1 mRNAs. We found that knockdown of DDX3 expression effectively inhibited HIV-1 production. Translation of HIV-1 early regulatory proteins, Tat and rev, was impaired in DDX3-depleted cells. All HIV-1 transcripts share a highly structured 5' untranslated region (UTR) with inhibitory elements on translation of viral mRNAs, yet DDX3 promoted translation of reporter mRNAs containing the HIV-1 5' UTR, especially with the transactivation response (TAR) hairpin. Interestingly, DDX3 directly interacts with HIV-1 Tat, a well-characterized transcriptional activator bound to the TAR hairpin. HIV-1 Tat is partially targeted to cytoplasmic stress granules upon DDX3 overexpression or cell stress conditions, suggesting a potential role of Tat/DDX3 complex in translation. We further demonstrated that HIV-1 Tat remains associated with translating mRNAs and facilitates translation of mRNAs containing the HIV-1 5' UTR. Taken together, these findings indicate that DDX3 is recruited to the TAR hairpin by interaction with viral Tat to facilitate HIV-1 mRNA translation.
Conflict of interest statement
Figures





Similar articles
-
DEAD-box RNA helicase DDX3 connects CRM1-dependent nuclear export and translation of the HIV-1 unspliced mRNA through its N-terminal domain.Biochim Biophys Acta. 2016 May;1859(5):719-30. doi: 10.1016/j.bbagrm.2016.03.009. Epub 2016 Mar 21. Biochim Biophys Acta. 2016. PMID: 27012366
-
DDX3 RNA helicase is required for HIV-1 Tat function.Biochem Biophys Res Commun. 2013 Nov 22;441(3):607-11. doi: 10.1016/j.bbrc.2013.10.107. Epub 2013 Oct 30. Biochem Biophys Res Commun. 2013. PMID: 24183723
-
Mechanism of HIV-1 Tat RNA translation and its activation by the Tat protein.Retrovirology. 2009 Aug 11;6:74. doi: 10.1186/1742-4690-6-74. Retrovirology. 2009. PMID: 19671151 Free PMC article.
-
DEAD-box RNA Helicase DDX3: Functional Properties and Development of DDX3 Inhibitors as Antiviral and Anticancer Drugs.Molecules. 2020 Feb 24;25(4):1015. doi: 10.3390/molecules25041015. Molecules. 2020. PMID: 32102413 Free PMC article. Review.
-
Focus on Translation Initiation of the HIV-1 mRNAs.Int J Mol Sci. 2018 Dec 28;20(1):101. doi: 10.3390/ijms20010101. Int J Mol Sci. 2018. PMID: 30597859 Free PMC article. Review.
Cited by
-
DDX RNA helicases: key players in cellular homeostasis and innate antiviral immunity.J Virol. 2024 Oct 22;98(10):e0004024. doi: 10.1128/jvi.00040-24. Epub 2024 Aug 30. J Virol. 2024. PMID: 39212449 Free PMC article. Review.
-
DDX3 functions in antiviral innate immunity through translational control of PACT.FEBS J. 2016 Jan;283(1):88-101. doi: 10.1111/febs.13553. Epub 2015 Oct 31. FEBS J. 2016. PMID: 26454002 Free PMC article.
-
DDX3 participates in miRNA biogenesis and RNA interference through translational control of PACT and interaction with AGO2.FEBS Open Bio. 2025 Jan;15(1):180-195. doi: 10.1002/2211-5463.13920. Epub 2024 Nov 14. FEBS Open Bio. 2025. PMID: 39543456 Free PMC article.
-
Dengue Virus Capsid Interacts with DDX3X-A Potential Mechanism for Suppression of Antiviral Functions in Dengue Infection.Front Cell Infect Microbiol. 2018 Jan 17;7:542. doi: 10.3389/fcimb.2017.00542. eCollection 2017. Front Cell Infect Microbiol. 2018. PMID: 29387631 Free PMC article.
-
HIV-1 replication and the cellular eukaryotic translation apparatus.Viruses. 2015 Jan 19;7(1):199-218. doi: 10.3390/v7010199. Viruses. 2015. PMID: 25606970 Free PMC article. Review.
References
-
- Chao CH, Chen CM, Cheng PL, Shih JW, Tsou AP et al. (2006) DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res 66: 6579-6588. doi:10.1158/0008-5472.CAN-05-2415. PubMed: 16818630. - DOI - PubMed
-
- Botlagunta M, Vesuna F, Mironchik Y, Raman A, Lisok A et al. (2008) Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27: 3912-3922. doi:10.1038/onc.2008.33. PubMed: 18264132. - DOI - PMC - PubMed
-
- Kanai Y, Dohmae N, Hirokawa N (2004) Kinesin transports RNA: isolation and characterization of an RNA-transporting granule. Neuron 43: 513-525. doi:10.1016/j.neuron.2004.07.022. PubMed: 15312650. - DOI - PubMed
-
- Choi YJ, Lee SG (2012) The DEAD-box RNA helicase DDX3 interacts with DDX5, co-localizes with it in the cytoplasm during the G2/M phase of the cycle, and affects its shuttling during mRNP export. J Cell Biochem 113: 985-996. doi:10.1002/jcb.23428. PubMed: 22034099. - DOI - PubMed
-
- Shih JW, Tsai TY, Chao CH, Wu Lee YH (2008) Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene 27: 700-714. doi:10.1038/sj.onc.1210687. PubMed: 17667941. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

