Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown
- PMID: 23840906
- PMCID: PMC3698076
- DOI: 10.1371/journal.pone.0069042
Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown
Abstract
Background: Intestinal barrier failure may lead to systemic inflammation and distant organ injury in patients following severe injury. Enteric glia cells (EGCs) have been shown to play an important role in maintaining gut barrier integrity through secretion of S-Nitrosoglutathione (GSNO). We have recently shown than Vagal Nerve Stimulation (VNS) increases EGC activation, which was associated with improved gut barrier integrity. Thus, we sought to further study the mechanism by which EGCs prevent intestinal barrier breakdown utilizing an in vitro model. We postulated that EGCs, through the secretion of GSNO, would improve intestinal barrier function through improved expression and localization of intestinal tight junction proteins.
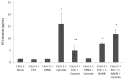
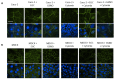
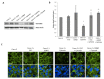
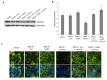
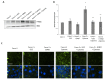
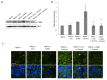
Methods: Epithelial cells were co-cultured with EGCs or incubated with GSNO and exposed to Cytomix (TNF-α, INF-γ, IL-1β) for 24 hours. Barrier function was assessed by permeability to 4kDa FITC-Dextran. Changes in tight junction proteins ZO-1, occludin, and phospho-MLC (P-MLC) were assessed by immunohistochemistry and immunoblot.
Key results: Co-culture of Cytomix-stimulated epithelial monolayers with EGCs prevented increases in permeability and improved expression and localization of occludin, ZO-1, and P-MLC. Further, treatment of epithelial monolayers with GSNO also prevented Cytomix-induced increases in permeability and exhibited a similar improvement in expression and localization of occludin, ZO-1, and P-MLC.
Conclusions & inferences: The addition of EGCs, or their secreted mediator GSNO, prevents epithelial barrier failure after injury and improved expression of tight junction proteins. Thus, therapies that increase EGC activation, such as VNS, may be a novel strategy to limit barrier failure in patients following severe injury.
Conflict of interest statement
Figures
References
-
- Musch MW, Walsh-Reitz MM, Chang EB (2006) Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol 290: G222-G231. doi:10.1152/ajpgi.00301.2005. PubMed: 16239402. - DOI - PubMed
-
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V et al. (2006) Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119: 2095-2106. doi:10.1242/jcs.02915. PubMed: 16638813. - DOI - PubMed
-
- Deitch EA, Shi HP, Lu Q, Feketeova E, Skurnick J et al. (2004) Mesenteric lymph from burned rats induces endothelial cell injury and activates neutrophils. Crit Care Med 32: 533-538. doi:10.1097/01.CCM.0000109773.00644.F4. PubMed: 14758175. - DOI - PubMed
-
- Krzyzaniak MJ, Peterson CY, Cheadle G, Loomis W, Wolf P et al. (2011) Efferent vagal nerve stimulation attenuates acute lung injury following burn: The importance of the gut-lung axis. Surgery 150: 379-389. doi:10.1016/j.surg.2011.06.008. PubMed: 21783215. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

