NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys
- PMID: 23840917
- PMCID: PMC3690252
- DOI: 10.1155/2013/462361
NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys
Abstract
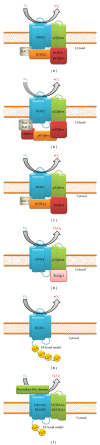
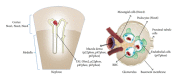
A major role of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase family of enzymes is to catalyze the production of superoxides and other reactive oxygen species (ROS). These ROS, in turn, play a key role as messengers in cell signal transduction and cell cycling, but when they are produced in excess they can lead to oxidative stress (OS). Oxidative stress in the kidneys is now considered a major cause of renal injury and inflammation, giving rise to a variety of pathological disorders. In this review, we discuss the putative role of oxalate in producing oxidative stress via the production of reactive oxygen species by isoforms of NADPH oxidases expressed in different cellular locations of the kidneys. Most renal cells produce ROS, and recent data indicate a direct correlation between upregulated gene expressions of NADPH oxidase, ROS, and inflammation. Renal tissue expression of multiple NADPH oxidase isoforms most likely will impact the future use of different antioxidants and NADPH oxidase inhibitors to minimize OS and renal tissue injury in hyperoxaluria-induced kidney stone disease.
Figures

References
-
- Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urological Research. 2005;33(5):349–357. - PubMed
-
- Holmes RP, Goodman HO, Assimos DG. Contribution of dietary oxalate to urinary oxalate excretion. Kidney International. 2001;59(1):270–276. - PubMed
-
- Holmes RP, Assimos DG. The impact of dietary oxalate on kidney stone formation. Urological Research. 2004;32(5):311–316. - PubMed
-
- Holmes RP, Ambrosius WT, Assimos DG. Dietary oxalate loads and renal oxalate handling. Journal of Urology. 2005;174(3):943–947. - PubMed
-
- Streit J, Tran-Ho LC, Königsberger E. Solubility of the three calcium oxalate hydrates in sodium chloride solutions and urine-like liquors. Monatshefte fur Chemie. 1998;129(12):1225–1236.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

