A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by G(αs) signaling
- PMID: 23840929
- PMCID: PMC3699835
- DOI: 10.7554/eLife.00782
A longitudinal study of Caenorhabditis elegans larvae reveals a novel locomotion switch, regulated by G(αs) signaling
Abstract
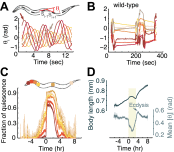
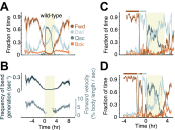
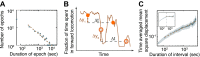
Despite their simplicity, longitudinal studies of invertebrate models are rare. We thus sought to characterize behavioral trends of Caenorhabditis elegans, from the mid fourth larval stage through the mid young adult stage. We found that, outside of lethargus, animals exhibited abrupt switching between two distinct behavioral states: active wakefulness and quiet wakefulness. The durations of epochs of active wakefulness exhibited non-Poisson statistics. Increased Gαs signaling stabilized the active wakefulness state before, during and after lethargus. In contrast, decreased Gαs signaling, decreased neuropeptide release, or decreased CREB activity destabilized active wakefulness outside of, but not during, lethargus. Taken together, our findings support a model in which protein kinase A (PKA) stabilizes active wakefulness, at least in part through two of its downstream targets: neuropeptide release and CREB. However, during lethargus, when active wakefulness is strongly suppressed, the native role of PKA signaling in modulating locomotion and quiescence may be minor. DOI:http://dx.doi.org/10.7554/eLife.00782.001.
Keywords: C. elegans; CREB; PKA; longitudinal study; modulation; quiet wakefulness; unc-31/CAPS.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

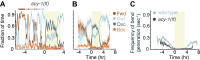
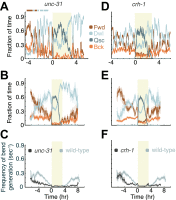
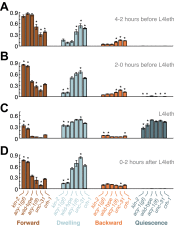

Similar articles
-
A wake-active locomotion circuit depolarizes a sleep-active neuron to switch on sleep.PLoS Biol. 2020 Feb 20;18(2):e3000361. doi: 10.1371/journal.pbio.3000361. eCollection 2020 Feb. PLoS Biol. 2020. PMID: 32078631 Free PMC article.
-
The microarchitecture of C. elegans behavior during lethargus: homeostatic bout dynamics, a typical body posture, and regulation by a central neuron.Sleep. 2013 Mar 1;36(3):385-95. doi: 10.5665/sleep.2456. Sleep. 2013. PMID: 23449971 Free PMC article.
-
The neuropeptide NLP-22 regulates a sleep-like state in Caenorhabditis elegans.Nat Commun. 2013;4:2846. doi: 10.1038/ncomms3846. Nat Commun. 2013. PMID: 24301180 Free PMC article.
-
A sleep state during C. elegans development.Curr Opin Neurobiol. 2013 Oct;23(5):824-30. doi: 10.1016/j.conb.2013.02.015. Epub 2013 Apr 3. Curr Opin Neurobiol. 2013. PMID: 23562486 Free PMC article. Review.
-
Call it Worm Sleep.Trends Neurosci. 2016 Feb;39(2):54-62. doi: 10.1016/j.tins.2015.12.005. Epub 2015 Dec 30. Trends Neurosci. 2016. PMID: 26747654 Free PMC article. Review.
Cited by
-
Food responsiveness regulates episodic behavioral states in Caenorhabditis elegans.J Neurophysiol. 2017 May 1;117(5):1911-1934. doi: 10.1152/jn.00555.2016. Epub 2017 Feb 22. J Neurophysiol. 2017. PMID: 28228583 Free PMC article.
-
Multi-well imaging of development and behavior in Caenorhabditis elegans.J Neurosci Methods. 2014 Feb 15;223:35-9. doi: 10.1016/j.jneumeth.2013.11.026. Epub 2013 Dec 7. J Neurosci Methods. 2014. PMID: 24321627 Free PMC article.
-
Serotonin promotes exploitation in complex environments by accelerating decision-making.BMC Biol. 2016 Feb 4;14:9. doi: 10.1186/s12915-016-0232-y. BMC Biol. 2016. PMID: 26847342 Free PMC article.
-
Distinct Mechanisms Underlie Quiescence during Two Caenorhabditis elegans Sleep-Like States.J Neurosci. 2015 Oct 28;35(43):14571-84. doi: 10.1523/JNEUROSCI.1369-15.2015. J Neurosci. 2015. PMID: 26511247 Free PMC article.
-
The Neuropeptides FLP-2 and PDF-1 Act in Concert To Arouse Caenorhabditis elegans Locomotion.Genetics. 2016 Nov;204(3):1151-1159. doi: 10.1534/genetics.116.192898. Epub 2016 Aug 31. Genetics. 2016. PMID: 27585848 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

