The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53
- PMID: 23841076
- PMCID: PMC3691929
- DOI: 10.1155/2013/568671
The omega-3 polyunsaturated fatty acid DHA induces simultaneous apoptosis and autophagy via mitochondrial ROS-mediated Akt-mTOR signaling in prostate cancer cells expressing mutant p53
Abstract
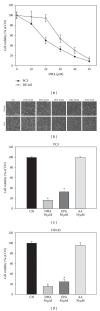
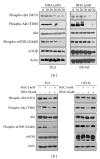
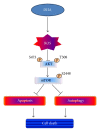
Docosahexaenoic acid (DHA) induces autophagy-associated apoptotic cell death in wild-type p53 cancer cells via regulation of p53. The present study investigated the effects of DHA on PC3 and DU145 prostate cancer cell lines harboring mutant p53. Results show that, in addition to apoptosis, DHA increased the expression levels of lipidated form LC3B and potently stimulated the autophagic flux, suggesting that DHA induces both autophagy and apoptosis in cancer cells expressing mutant p53. DHA led to the generation of mitochondrial reactive oxygen species (ROS), as shown by the mitochondrial ROS-specific probe mitoSOX. Similarly, pretreatment with the antioxidant N-acetyl-cysteine (NAC) markedly inhibited both the autophagy and the apoptosis triggered by DHA, indicating that mitochondrial ROS mediate the cytotoxicity of DHA in mutant p53 cells. Further, DHA reduced the levels of phospho-Akt and phospho-mTOR in a concentration-dependent manner, while NAC almost completely blocked that effect. Collectively, these findings present a novel mechanism of ROS-regulated apoptosis and autophagy that involves Akt-mTOR signaling in prostate cancer cells with mutant p53 exposed to DHA.
Figures


Similar articles
-
Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53.Autophagy. 2011 Nov;7(11):1348-58. doi: 10.4161/auto.7.11.16658. Epub 2011 Nov 1. Autophagy. 2011. PMID: 21811093 Free PMC article.
-
Coptisine induces autophagic cell death through down-regulation of PI3K/Akt/mTOR signaling pathway and up-regulation of ROS-mediated mitochondrial dysfunction in hepatocellular carcinoma Hep3B cells.Arch Biochem Biophys. 2021 Jan 15;697:108688. doi: 10.1016/j.abb.2020.108688. Epub 2020 Nov 21. Arch Biochem Biophys. 2021. PMID: 33227289
-
Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway.Toxicol Appl Pharmacol. 2016 Aug 1;304:59-69. doi: 10.1016/j.taap.2016.05.018. Epub 2016 May 25. Toxicol Appl Pharmacol. 2016. PMID: 27236003
-
p53-Mediated Molecular Control of Autophagy in Tumor Cells.Biomolecules. 2018 Mar 21;8(2):14. doi: 10.3390/biom8020014. Biomolecules. 2018. PMID: 29561758 Free PMC article. Review.
-
p53 and metabolism: from mechanism to therapeutics.Oncotarget. 2018 May 4;9(34):23780-23823. doi: 10.18632/oncotarget.25267. eCollection 2018 May 4. Oncotarget. 2018. PMID: 29805774 Free PMC article. Review.
Cited by
-
Docoxahexaenoic Acid Induces Apoptosis of Pancreatic Cancer Cells by Suppressing Activation of STAT3 and NF-κB.Nutrients. 2018 Nov 2;10(11):1621. doi: 10.3390/nu10111621. Nutrients. 2018. PMID: 30400136 Free PMC article.
-
Inhibitors of the PI3K/Akt/mTOR Pathway in Prostate Cancer Chemoprevention and Intervention.Pharmaceutics. 2021 Aug 3;13(8):1195. doi: 10.3390/pharmaceutics13081195. Pharmaceutics. 2021. PMID: 34452154 Free PMC article. Review.
-
Omega-3 Polyunsaturated Fatty Acids May Attenuate Streptozotocin-Induced Pancreatic β-Cell Death via Autophagy Activation in Fat1 Transgenic Mice.Endocrinol Metab (Seoul). 2015 Dec;30(4):569-75. doi: 10.3803/EnM.2015.30.4.569. Endocrinol Metab (Seoul). 2015. PMID: 26790385 Free PMC article.
-
Iron oxide magnetic nanoparticles combined with actein suppress non-small-cell lung cancer growth in a p53-dependent manner.Int J Nanomedicine. 2017 Oct 17;12:7627-7651. doi: 10.2147/IJN.S127549. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29089760 Free PMC article.
-
Beneficial Effects of a Mixture of Algae and Extra Virgin Olive Oils on the Age-Induced Alterations of Rodent Skeletal Muscle: Role of HDAC-4.Nutrients. 2020 Dec 25;13(1):44. doi: 10.3390/nu13010044. Nutrients. 2020. PMID: 33375628 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer Journal for Clinicians. 2012;62(1):10–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

