Zn(II) ions substantially perturb Cu(II) ion coordination in amyloid-β at physiological pH
- PMID: 23841511
- PMCID: PMC3775258
- DOI: 10.1021/jp406067n
Zn(II) ions substantially perturb Cu(II) ion coordination in amyloid-β at physiological pH
Abstract
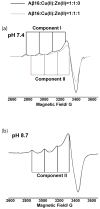
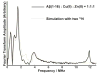
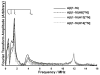
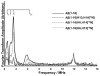
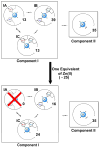
The interaction of Cu(II) and Zn(II) ions with amyloid-β (Aβ) plays an important role in the etiology of Alzheimer's disease. We describe the use of electron spin resonance (ESR) to measure metal-binding competition between Cu(II) and Zn(II) in amyloid-β at physiological pH. Continuous wave ESR measurements show that the affinity of Cu(II) toward Aβ(1-16) is significantly higher than that of Zn(II) at physiological pH. Importantly, of the two known Cu(II) coordination modes in Aβ, component I and component II, Zn(II) displaces Cu(II) only from component I. Our results indicate that at excess amounts of Zn(II) component II becomes the most dominant coordination mode. This observation is important as Aβ aggregates in the brain contain a high Zn(II) ion concentration. In order to determine details of the metal ion competition, electron spin echo envelope modulation experiments were carried out on Aβ variants that were systematically (15)N labeled. In the presence of Zn(II), most peptides use His 14 as an equatorial ligand to bind Cu(II) ions. Interestingly, Zn(II) ions completely substitute Cu(II) ions that are simultaneously coordinated to His 6 and His 13. Furthermore, in the presence of Zn(II), the proportion of Cu(II) ions that are simultaneously coordinated to His 13 and His 14 is increased. On the basis of our results we suggest that His 13 plays a critical role in modulating the morphology of Aβ aggregates.
Figures



Similar articles
-
ESEEM analysis of multi-histidine Cu(II)-coordination in model complexes, peptides, and amyloid-β.J Phys Chem B. 2014 Jul 31;118(30):8935-44. doi: 10.1021/jp500767n. Epub 2014 Jul 22. J Phys Chem B. 2014. PMID: 25014537 Free PMC article.
-
Metal ions and intrinsically disordered proteins and peptides: from Cu/Zn amyloid-β to general principles.Acc Chem Res. 2014 Aug 19;47(8):2252-9. doi: 10.1021/ar400293h. Epub 2014 May 29. Acc Chem Res. 2014. PMID: 24871565
-
Substantial contribution of the two imidazole rings of the His13-His14 dyad to Cu(II) binding in amyloid-β(1-16) at physiological pH and its significance.J Phys Chem A. 2011 Sep 1;115(34):9590-602. doi: 10.1021/jp200379m. Epub 2011 Apr 14. J Phys Chem A. 2011. PMID: 21491887 Free PMC article.
-
Cu and Zn interactions with Aβ peptides: consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers.Metallomics. 2019 Jan 23;11(1):64-84. doi: 10.1039/c8mt00203g. Metallomics. 2019. PMID: 30234208 Review.
-
Mutual interference of Cu and Zn ions in Alzheimer's disease: perspectives at the molecular level.Dalton Trans. 2017 Oct 14;46(38):12750-12759. doi: 10.1039/c7dt01344b. Epub 2017 Sep 22. Dalton Trans. 2017. PMID: 28937157 Free PMC article. Review.
Cited by
-
Copper-zinc cross-modulation in prion protein binding.Eur Biophys J. 2014 Dec;43(12):631-42. doi: 10.1007/s00249-014-0993-6. Epub 2014 Nov 14. Eur Biophys J. 2014. PMID: 25395329 Free PMC article.
-
Amyloid Oligomers: A Joint Experimental/Computational Perspective on Alzheimer's Disease, Parkinson's Disease, Type II Diabetes, and Amyotrophic Lateral Sclerosis.Chem Rev. 2021 Feb 24;121(4):2545-2647. doi: 10.1021/acs.chemrev.0c01122. Epub 2021 Feb 5. Chem Rev. 2021. PMID: 33543942 Free PMC article. Review.
-
Kinetic Analysis Reveals the Identity of Aβ-Metal Complex Responsible for the Initial Aggregation of Aβ in the Synapse.ACS Chem Neurosci. 2017 Sep 20;8(9):1970-1979. doi: 10.1021/acschemneuro.7b00121. Epub 2017 Jun 29. ACS Chem Neurosci. 2017. PMID: 28621929 Free PMC article.
-
Copper(II) Can Kinetically Trap Arctic and Italian Amyloid-β40 as Toxic Oligomers, Mimicking Cu(II) Binding to Wild-Type Amyloid-β42: Implications for Familial Alzheimer's Disease.JACS Au. 2024 Feb 6;4(2):578-591. doi: 10.1021/jacsau.3c00687. eCollection 2024 Feb 26. JACS Au. 2024. PMID: 38425915 Free PMC article.
-
Cu2+-based distance measurements by pulsed EPR provide distance constraints for DNA backbone conformations in solution.Nucleic Acids Res. 2020 May 21;48(9):e49. doi: 10.1093/nar/gkaa133. Nucleic Acids Res. 2020. PMID: 32095832 Free PMC article.
References
-
- Maret W. Metalloproteomics, Metalloproteomes, and the Annotation of Metalloproteins. Metallomics. 2010;2:117–125. - PubMed
-
- Kim BE, Nevitt T, Thiele DJ. Mechanisms for Copper Acquisition, Distribution and Regulation. Nat Chem Biol. 2008;4:176–185. - PubMed
-
- Ducea JA, Bush AI. Biological Metals and Alzheimer’s Disease: Implications for Therapeutics and Diagnostics. Progress in Neurobiology. 2010;92:1–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

