Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis
- PMID: 23841633
- PMCID: PMC3867987
- DOI: 10.2217/fmb.13.52
Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis
Abstract
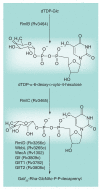
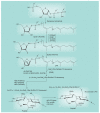
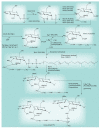
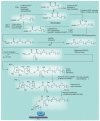
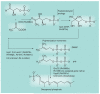
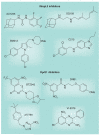
Most of the newly discovered compounds showing promise for the treatment of TB, notably multidrug-resistant TB, inhibit aspects of Mycobacterium tuberculosis cell envelope metabolism. This review reflects on the evolution of the knowledge that many of the front-line and emerging products inhibit aspects of cell envelope metabolism and in the process are bactericidal not only against actively replicating M. tuberculosis, but contrary to earlier impressions, are effective against latent forms of the disease. While mycolic acid and arabinogalactan synthesis are still primary targets of existing and new drugs, peptidoglycan synthesis, transport mechanisms and the synthesis of the decaprenyl-phosphate carrier lipid all show considerable promise as targets for new products, older drugs and new combinations. The advantages of whole cell- versus target-based screening in the perpetual search for new targets and products to counter multidrug-resistant TB are discussed.
Figures


Similar articles
-
Arabinogalactan and lipoarabinomannan biosynthesis: structure, biogenesis and their potential as drug targets.Future Microbiol. 2012 Jan;7(1):129-47. doi: 10.2217/fmb.11.123. Future Microbiol. 2012. PMID: 22191451 Review.
-
Targeting the formation of the cell wall core of M. tuberculosis.Infect Disord Drug Targets. 2007 Jun;7(2):182-202. doi: 10.2174/187152607781001808. Infect Disord Drug Targets. 2007. PMID: 17970228 Free PMC article. Review.
-
Emerging therapeutic targets in tuberculosis: post-genomic era.Expert Opin Ther Targets. 2002 Feb;6(1):21-40. doi: 10.1517/14728222.6.1.21. Expert Opin Ther Targets. 2002. PMID: 11901479 Review.
-
Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis.Adv Appl Microbiol. 2009;69:23-78. doi: 10.1016/S0065-2164(09)69002-X. Adv Appl Microbiol. 2009. PMID: 19729090 Free PMC article. Review.
-
The DprE1 enzyme, one of the most vulnerable targets of Mycobacterium tuberculosis.Appl Microbiol Biotechnol. 2013 Oct;97(20):8841-8. doi: 10.1007/s00253-013-5218-x. Epub 2013 Sep 14. Appl Microbiol Biotechnol. 2013. PMID: 24037308 Review.
Cited by
-
Molecular docking studies on InhA, MabA and PanK enzymes from Mycobacterium tuberculosis of ellagic acid derivatives from Ludwigia adscendens and Trewia nudiflora.In Silico Pharmacol. 2015 Dec;3(1):10. doi: 10.1186/s40203-015-0014-1. Epub 2015 Dec 8. In Silico Pharmacol. 2015. PMID: 26820895 Free PMC article.
-
New Insights in to the Intrinsic and Acquired Drug Resistance Mechanisms in Mycobacteria.Front Microbiol. 2017 Apr 25;8:681. doi: 10.3389/fmicb.2017.00681. eCollection 2017. Front Microbiol. 2017. PMID: 28487675 Free PMC article. Review.
-
RNA-Seq analysis uncovers non-coding small RNA system of Mycobacterium neoaurum in the metabolism of sterols to accumulate steroid intermediates.Microb Cell Fact. 2016 Apr 25;15:64. doi: 10.1186/s12934-016-0462-2. Microb Cell Fact. 2016. PMID: 27112590 Free PMC article.
-
The Redox State Regulates the Conformation of Rv2466c to Activate the Antitubercular Prodrug TP053.J Biol Chem. 2015 Dec 25;290(52):31077-89. doi: 10.1074/jbc.M115.677039. Epub 2015 Nov 6. J Biol Chem. 2015. PMID: 26546681 Free PMC article.
-
New antituberculous drugs derived from natural products: current perspectives and issues in antituberculous drug development.J Antibiot (Tokyo). 2017 Nov 1. doi: 10.1038/ja.2017.126. Online ahead of print. J Antibiot (Tokyo). 2017. PMID: 29089593 Review.
References
-
- Center for Disease Control and Prevention: emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs worldwide, 2000-2004. MMWR Morb. Mortal. Wkly Rep. 2006;55(11):301–305. - PubMed
-
- Bhamidi S, Shi L, Chatterjee D, Belisle JT, Crick DC, McNeil MR. A bioanalytical method to determine the cell wall composition of Mycobacterium tuberculosis grown in vivo. Anal. Biochem. 2012;421(1):240–249. - PubMed
Websites
-
- WHO Tuberculosis. www.who.int/mediacentre/factsheets/fs104/en.
-
- PubChem BioAssay Inhibitors of mycobacterial glucosamine-1-phosphate acetyl transferase (GlmU) – bioassay summary. http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1376.
-
- Working Group on New TB Drugs Stop TB Partnership. www.newtbdrugs.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
