Deleted in breast cancer-1 (DBC-1) in the interface between metabolism, aging and cancer
- PMID: 23841676
- PMCID: PMC3755336
- DOI: 10.1042/BSR20130062
Deleted in breast cancer-1 (DBC-1) in the interface between metabolism, aging and cancer
Abstract
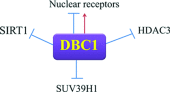
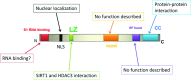
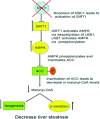
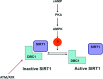
DBC1 (deleted in breast cancer-1) is a nuclear protein that regulates cellular metabolism. Since alteration in cellular metabolism have been proposed to be the emerging 'hallmark' of cancer, it is possible that DBC1 may be implicated in the regulation of cancer cell energy metabolism. However, at this point any role of DBC1 in cancer is only speculative. In this review, we will discuss the new developments in DBC1 research, its molecular structure, regulatory roles and implication in metabolism, aging and cancer.
Figures
Similar articles
-
Advances on the role of the deleted in breast cancer (DBC1) in cancer and autoimmune diseases.J Leukoc Biol. 2021 Feb;109(2):449-454. doi: 10.1002/JLB.6MR0320-086R. Epub 2020 Apr 26. J Leukoc Biol. 2021. PMID: 32337788 Review.
-
HDAC3 is negatively regulated by the nuclear protein DBC1.J Biol Chem. 2010 Dec 24;285(52):40830-7. doi: 10.1074/jbc.M110.153270. Epub 2010 Oct 28. J Biol Chem. 2010. PMID: 21030595 Free PMC article.
-
Histone acetylation in gene regulation.Brief Funct Genomic Proteomic. 2006 Sep;5(3):209-21. doi: 10.1093/bfgp/ell028. Epub 2006 Jul 28. Brief Funct Genomic Proteomic. 2006. PMID: 16877467 Review.
-
Histone deacetylases and cancer.Oncogene. 2007 Aug 13;26(37):5420-32. doi: 10.1038/sj.onc.1210610. Oncogene. 2007. PMID: 17694083 Review.
-
Deleted in breast cancer 1 (DBC1) is a dynamically regulated protein.Neoplasma. 2010;57(4):365-8. Neoplasma. 2010. PMID: 20429629
Cited by
-
A novel form of Deleted in breast cancer 1 (DBC1) lacking the N-terminal domain does not bind SIRT1 and is dynamically regulated in vivo.Sci Rep. 2019 Oct 7;9(1):14381. doi: 10.1038/s41598-019-50789-7. Sci Rep. 2019. PMID: 31591441 Free PMC article.
-
Deleted in breast cancer 1 (DBC1) protein regulates hepatic gluconeogenesis.J Biol Chem. 2014 Feb 28;289(9):5518-27. doi: 10.1074/jbc.M113.512913. Epub 2014 Jan 10. J Biol Chem. 2014. PMID: 24415752 Free PMC article.
-
CCAR2 Is Required for Proliferation and Tumor Maintenance in Human Squamous Cell Carcinoma.J Invest Dermatol. 2017 Feb;137(2):506-512. doi: 10.1016/j.jid.2016.09.027. Epub 2016 Oct 7. J Invest Dermatol. 2017. PMID: 27725203 Free PMC article.
-
Mechanistic insights into the dual role of CCAR2/DBC1 in cancer.Exp Mol Med. 2023 Aug;55(8):1691-1701. doi: 10.1038/s12276-023-01058-1. Epub 2023 Aug 1. Exp Mol Med. 2023. PMID: 37524873 Free PMC article. Review.
-
Mechanisms of Artemisia scoparia's Anti-Inflammatory Activity in Cultured Adipocytes, Macrophages, and Pancreatic β-Cells.Obesity (Silver Spring). 2020 Sep;28(9):1726-1735. doi: 10.1002/oby.22912. Epub 2020 Aug 2. Obesity (Silver Spring). 2020. PMID: 32741148 Free PMC article.
References
-
- Di Marcotullio L., Canettieri G., Infante P., Greco A., Gulino A. Protected from the inside: endogenous histone deacetylase inhibitors and the road to cancer. Biochim. Biophys. Acta. 2011;1815:241–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

