Ameloblasts require active RhoA to generate normal dental enamel
- PMID: 23841780
- PMCID: PMC3711190
- DOI: 10.1111/eos.12059
Ameloblasts require active RhoA to generate normal dental enamel
Abstract
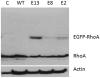
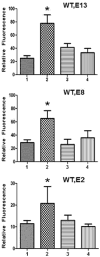
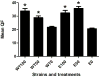
RhoA plays a fundamental role in regulation of the actin cytoskeleton, intercellular attachment, and cell proliferation. During amelogenesis, ameloblasts (which produce the enamel proteins) undergo dramatic cytoskeletal changes and the RhoA protein level is up-regulated. Transgenic mice were generated that express a dominant-negative RhoA transgene in ameloblasts using amelogenin gene-regulatory sequences. Transgenic and wild-type (WT) molar tooth germs were incubated with sodium fluoride (NaF) or sodium chloride (NaCl) in organ culture. Filamentous actin (F-actin) stained with phalloidin was elevated significantly in WT ameloblasts treated with NaF compared with WT ameloblasts treated with NaCl or with transgenic ameloblasts treated with NaF, thereby confirming a block in the RhoA/Rho-associated protein kinase (ROCK) pathway in the transgenic mice. Little difference in quantitative fluorescence (an estimation of fluorosis) was observed between WT and transgenic incisors from mice provided with drinking water containing NaF. We subsequently found reduced transgene expression in incisors compared with molars. Transgenic molar teeth had reduced amelogenin, E-cadherin, and Ki67 compared with WT molar teeth. Hypoplastic enamel in transgenic mice correlates with reduced expression of the enamel protein, amelogenin, and E-cadherin and cell proliferation are regulated by RhoA in other tissues. Together these findings reveal deficits in molar ameloblast function when RhoA activity is inhibited.
Keywords: ameloblasts; dental enamel; dominant-negative RhoA; transgenic mice.
© 2013 Eur J Oral Sci.
Conflict of interest statement
This work did not involve any conflicts of interest.
Figures






References
-
- THESLEFF I, SHARPE P. Signaling networks regulating dental development. Mech Dev. 1997;67:111–123. - PubMed
-
- JERNVALL J, THESLEFF I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. - PubMed
-
- SMITH CE, NANCI A. Overview of morphological changes in enamel organ cells associated with major events in amelogenesis. Int J Dev Biol. 1995;39:153–161. - PubMed
-
- SMITH CE. Ameloblasts: secretory and resorptive functions. J Dent Res. 1979;58(Spec Issue B):695–707. - PubMed
-
- SMITH CE. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 1998;9:128–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

