Early olfactory processing in Drosophila: mechanisms and principles
- PMID: 23841839
- PMCID: PMC3933953
- DOI: 10.1146/annurev-neuro-062111-150533
Early olfactory processing in Drosophila: mechanisms and principles
Abstract
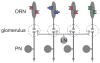
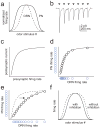
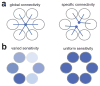
In the olfactory system of Drosophila melanogaster, it is relatively straightforward to target in vivo measurements of neural activity to specific processing channels. This, together with the numerical simplicity of the Drosophila olfactory system, has produced rapid gains in our understanding of Drosophila olfaction. This review summarizes the neurophysiology of the first two layers of this system: the peripheral olfactory receptor neurons and their postsynaptic targets in the antennal lobe. We now understand in some detail the cellular and synaptic mechanisms that shape odor representations in these neurons. Together, these mechanisms imply that interesting neural adaptations to environmental statistics have occurred. These mechanisms also place some fundamental constraints on early sensory processing that pose challenges for higher brain regions. These findings suggest some general principles with broad relevance to early sensory processing in other modalities.
Figures
Similar articles
-
Transformation of olfactory representations in the Drosophila antennal lobe.Science. 2004 Jan 16;303(5656):366-70. doi: 10.1126/science.1090782. Epub 2003 Dec 18. Science. 2004. PMID: 14684826
-
Molecular, anatomical, and functional organization of the Drosophila olfactory system.Curr Biol. 2005 Sep 6;15(17):1535-47. doi: 10.1016/j.cub.2005.07.034. Curr Biol. 2005. PMID: 16139208
-
Internal representations of smell in the Drosophila brain.J Biomed Sci. 2007 Jul;14(4):453-9. doi: 10.1007/s11373-007-9168-0. Epub 2007 Apr 14. J Biomed Sci. 2007. PMID: 17440836 Review.
-
Dystrophin is required for normal synaptic gain in the Drosophila olfactory circuit.Brain Res. 2019 Jun 1;1712:158-166. doi: 10.1016/j.brainres.2019.01.039. Epub 2019 Jan 31. Brain Res. 2019. PMID: 30711401
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
Cited by
-
Developmental experience-dependent plasticity in the first synapse of the Drosophila olfactory circuit.J Neurophysiol. 2016 Dec 1;116(6):2730-2738. doi: 10.1152/jn.00616.2016. Epub 2016 Sep 28. J Neurophysiol. 2016. PMID: 27683892 Free PMC article. Review.
-
Candidate chemosensory receptors in the antennae and maxillae of Spodoptera frugiperda (J. E. Smith) larvae.Front Physiol. 2022 Sep 15;13:970915. doi: 10.3389/fphys.2022.970915. eCollection 2022. Front Physiol. 2022. PMID: 36187799 Free PMC article.
-
How the insect central complex could coordinate multimodal navigation.Elife. 2021 Dec 9;10:e73077. doi: 10.7554/eLife.73077. Elife. 2021. PMID: 34882094 Free PMC article.
-
Functional morphology of the primary olfactory centers in the brain of the hermit crab Coenobita clypeatus (Anomala, Coenobitidae).Cell Tissue Res. 2020 Jun;380(3):449-467. doi: 10.1007/s00441-020-03199-5. Epub 2020 Apr 2. Cell Tissue Res. 2020. PMID: 32242250 Free PMC article.
-
Effects of stochastic coding on olfactory discrimination in flies and mice.PLoS Biol. 2023 Oct 31;21(10):e3002206. doi: 10.1371/journal.pbio.3002206. eCollection 2023 Oct. PLoS Biol. 2023. PMID: 37906721 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

