Deconstructing activation events in rhodopsin
- PMID: 23841875
- PMCID: PMC3774788
- DOI: 10.1021/ja4042687
Deconstructing activation events in rhodopsin
Abstract
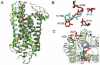
Activation of class-A G-protein-coupled receptors (GPCRs) involves large-scale reorganization of the H3/H6 interhelical network. In rhodopsin (Rh), this process is coupled to a change in the protonation state of a key residue, E134, whose exact role in activation is not well understood. Capturing this millisecond pH-dependent process is a well-appreciated challenge. We have developed a scheme combining the harmonic Fourier beads (HFB) method and constant-pH molecular dynamics with pH-based replica exchange (pH-REX) to gain insight into the structural changes that occur along the activation pathway as a function of the protonation state of E134. Our results indicate that E134 is protonated as a consequence of tilting of H6 by ca. 4.0° with respect to its initial position and simultaneous rotation by ca. 23° along its principal axis. The movement of H6 is associated with breakage of the E247-R135 and R135-E134 salt bridges and concomitant release of the E134 side chain, which results in an increase in its pKa value above physiological pH. An increase in the hydrophobicity of the environment surrounding E134 leads to further tilting and rotation of H6 and upshift of the E134 pKa. Such atomic-level information, which is not accessible through experiments, refines the earlier proposed sequential model of Rh activation (see: Zaitseva, E.; et al. Sequential Rearrangement of Interhelical Networks Upon Rhodopsin Activation in Membranes: The Meta IIa Conformational Substate . J. Am. Chem. Soc. 2010, 132, 4815) and argues that the E134 protonation switch is both a cause and a consequence of the H6 motion.
Figures
References
-
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Nature. 2013;494:185–194. - PubMed
-
- Deupi X, Standfuss J. Curr. Opin. Struct. Biol. 2011;21:541–551. - PubMed
-
- Hubbell WL, Altenbach C, Hubbell CM, Khorana HG. In: Membrane Proteins. Chemistry DCRBT-A, P., editors. Volume 63. Academic Press; 2003. pp. 243–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

