Guidelines for guideline developers: a systematic review of grading systems for medical tests
- PMID: 23842037
- PMCID: PMC3716938
- DOI: 10.1186/1748-5908-8-78
Guidelines for guideline developers: a systematic review of grading systems for medical tests
Abstract
Background: A variety of systems have been developed to grade evidence and develop recommendations based on the available evidence. However, development of guidelines for medical tests is especially challenging given the typical indirectness of the evidence; direct evidence of the effects of testing on patient important outcomes is usually absent. We compared grading systems for medical tests on how they use evidence in guideline development.
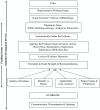
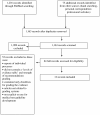
Methods: We used a systematic strategy to look for grading systems specific to medical tests in PubMed, professional guideline websites, via personal correspondence, and handsearching back references of key articles. Using the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument as a starting point, we defined two sets of characteristics to describe these systems: methodological and process ones. Methodological characteristics are features relating to how evidence is gathered, appraised, and used in recommendations. Process characteristics are those relating to the guideline development process. Data were extracted in duplicate and differences resolved through discussion.
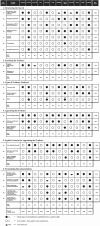
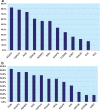
Results: Twelve grading systems could be included. All varied in the degree to which methodological and process characteristics were addressed. Having a clinical scenario, identifying the care pathway and/or developing an analytical framework, having explicit criteria for appraising and linking indirect evidence, and having explicit methodologies for translating evidence into recommendations were least frequently addressed. Five systems at most addressed these, to varying degrees of explicitness and completeness. Process wise, features most frequently addressed included involvement of relevant professional groups (8/12), external peer review of completed guidelines (9/12), and recommendations on methods for dissemination (8/12). Characteristics least often addressed were whether the system was piloted (3/12) and funder information (3/12).
Conclusions: Five systems for grading evidence about medical tests in guideline development addressed to differing degrees of explicitness the need for and appraisal of different bodies of evidence, the linking of such evidence, and its translation into recommendations. At present, no one system addressed the full complexity of gathering, assessing and linking different bodies of evidence.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
The educational effects of portfolios on undergraduate student learning: a Best Evidence Medical Education (BEME) systematic review. BEME Guide No. 11.Med Teach. 2009 Apr;31(4):282-98. doi: 10.1080/01421590902889897. Med Teach. 2009. PMID: 19404891
-
Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain.Spine (Phila Pa 1976). 2011 Oct 1;36(21 Suppl):S144-63. doi: 10.1097/BRS.0b013e31822ef5b4. Spine (Phila Pa 1976). 2011. PMID: 21952186
-
Health professionals' experience of teamwork education in acute hospital settings: a systematic review of qualitative literature.JBI Database System Rev Implement Rep. 2016 Apr;14(4):96-137. doi: 10.11124/JBISRIR-2016-1843. JBI Database System Rev Implement Rep. 2016. PMID: 27532314
-
Incorporating considerations of cost-effectiveness, affordability, and resource implications in guideline development: article 6 in Integrating and coordinating efforts in COPD guideline development. An official ATS/ERS workshop report.Proc Am Thorac Soc. 2012 Dec;9(5):251-5. doi: 10.1513/pats.201208-059ST. Proc Am Thorac Soc. 2012. PMID: 23256167
Cited by
-
Are Guidelines Guiding us on How to Utilize Laboratory Tests?EJIFCC. 2015 Aug 24;26(3):146-57. eCollection 2015 Aug. EJIFCC. 2015. PMID: 27683490 Free PMC article.
-
What's in a Guideline? Developing Collaborative and Sound Research Designs that Substantiate Best Practice Recommendations for Transgender Health Care.AMA J Ethics. 2016 Nov 1;18(11):1098-1106. doi: 10.1001/journalofethics.2016.18.11.stas1-1611. AMA J Ethics. 2016. PMID: 27883301 Free PMC article.
-
Application of the GRADE Approach in the Development of Guidelines and Recommendations in Genomic Medicine.Genomics Insights. 2018 Jan 30;11:1178631017753360. doi: 10.1177/1178631017753360. eCollection 2018. Genomics Insights. 2018. PMID: 29410601 Free PMC article.
-
Evidence synthesis and guideline development in genomic medicine: current status and future prospects.Genet Med. 2015 Jan;17(1):63-7. doi: 10.1038/gim.2014.69. Epub 2014 Jun 19. Genet Med. 2015. PMID: 24946156 Free PMC article.
-
Exploring the Evidence Base Behind Quality Measures.Am J Med Qual. 2018 May/Jun;33(3):321-322. doi: 10.1177/1062860617721645. Epub 2017 Jul 24. Am J Med Qual. 2018. PMID: 28737042 Free PMC article. No abstract available.
References
-
- Atkins D, Eccles M, Flottorp S, Guyatt GH, Henry D, Hill S, Liberati A, O’Connell D, Oxman AD, Phillips B, Schünemann H, Edejer TT, Vist GE, Williams JW Jr. The GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches. BMC Health Serv Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. - DOI - PMC - PubMed
-
- West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, Lux L. Systems to rate the strength of scientific evidence. Evidence Report/Technology Assessment No. 47 (Prepared by the Research Triangle Institute-University of North Carolina Evidence-based Practice Center under Contract No. 290-97-0011). In AHRQ Publication No. 02-E016. Agency for Healthcare Research and Quality: Rockville, MD; 2002.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources