Intracranial electrical impedance tomography: a method of continuous monitoring in an animal model of head trauma
- PMID: 23842194
- PMCID: PMC3783592
- DOI: 10.1213/ANE.0b013e318290c7b7
Intracranial electrical impedance tomography: a method of continuous monitoring in an animal model of head trauma
Abstract
Background: Electrical impedance tomography (EIT) is a method that can render continuous graphical cross-sectional images of the brain's electrical properties. Because these properties can be altered by variations in water content, shifts in sodium concentration, bleeding, and mass deformation, EIT has promise as a sensitive instrument for head injury monitoring to improve early recognition of deterioration and to observe the benefits of therapeutic intervention. This study presents a swine model of head injury used to determine the detection capabilities of an inexpensive bedside EIT monitoring system with a novel intracranial pressure (ICP)/EIT electrode combination sensor on induced intraparenchymal mass effect, intraparenchymal hemorrhage, and cessation of brain blood flow. Conductivity difference images are shown in conjunction with ICP data, confirming the effects.
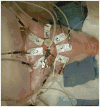
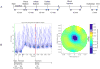
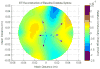
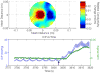
Methods: Eight domestic piglets (3-4 weeks of age, mean 10 kg), under general anesthesia, were subjected to 4 injuries: induced intraparenchymal mass effect using an inflated, and later, deflated 0.15-mL Fogarty catheter; hemorrhage by intraparenchymal injection of 1-mL arterial blood; and ischemia/infarction by euthanasia. EIT and ICP data were recorded 10 minutes before inducing the injury until 10 minutes after injury. Continuous EIT and ICP monitoring were facilitated by a ring of circumferentially disposed cranial Ag/AgCl electrodes and 1 intraparenchymal ICP/EIT sensor electrode combination. Data were recorded at 100 Hz. Two-dimensional tomographic conductivity difference (Δσ) images, rendered using data before and after an injury, were displayed in real time on an axial circular mesh. Regions of interest (ROI) within the images were automatically selected as the upper or lower 5% of conductivity data depending on the nature of the injury. Mean Δσ within the ROIs and background were statistically analyzed. ROI Δσ was compared with the background Δσ after an injury event using an unpaired, unequal variance t test. Conductivity change within an ROI after injury was likewise compared with the same ROI before the injury making use of unpaired t tests with unequal variance.
Results: Eight animal subjects were studied, each undergoing 4 injury events including euthanasia. Changes in conductivity due to injury showed expected pathophysiologic effects in an ROI identified within the middle of the left hemisphere; this localization is reasonable given the actual site of injury (left hemisphere) and spatial warping associated with estimating a 3-dimensional conductivity distribution in 2-dimensional space. Results are shown as mean ± 1 SD. When averaged across all 8 animals, balloon inflation caused the mean Δσ within the ROI to shift by -11.4 ± 10.9 mS/m; balloon deflation by +9.4 ± 8.8 mS/m; blood injection by +19.5 ± 11.5 mS/m; death by -12.6 ± 13.2 mS/m. All induced injuries were detectable to statistical significance (P < 0.0001).
Conclusion: This study confirms that the bedside EIT system with ICP/EIT combination sensor can detect induced trauma. Such a technique may hold promise for further research in the monitoring and management of traumatically brain-injured individuals.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Metherall P, Barber DC, Smallwood RH, Brown BH. Three dimensional electrical impedance tomography. Nature. 1996;380(6574):509–12. - PubMed
-
- Bayford R, Tizzard A. Bioimpedance imaging: an overview of potential clinical applications. Analyst. 2012;137(20):4635–43. - PubMed
-
- Holder D. Methods, History, and Applications. London: The Institute of Physics Publishing; 2005. Electrical Impedance Tomography.
-
- da Silva JE, de Sá JP, Jossinet J. Classification of breast tissue by electrical impedance spectroscopy. Med Biol Eng Comput. 2000;38(1):26–30. - PubMed
-
- Poplack SP, Tosteson TD, Wells WA, Pogue BW, Meaney PM, Hartov A, Kogel CA, Soho SK, Gibson JJ, Paulsen KD. Electromagnetic Breast Imaging: Results of a Pilot Study in Women. Radiology. 2007;243(2):350–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

