Rationale, timeline, study design, and protocol overview of the therapeutic hypothermia after pediatric cardiac arrest trials
- PMID: 23842585
- PMCID: PMC3947631
- DOI: 10.1097/PCC.0b013e31828a863a
Rationale, timeline, study design, and protocol overview of the therapeutic hypothermia after pediatric cardiac arrest trials
Abstract
Objective: To describe the rationale, timeline, study design, and protocol overview of the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials.
Design: Multicenter randomized controlled trials.
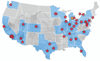
Setting: Pediatric intensive care and cardiac ICUs in the United States and Canada.
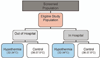
Patients: Children from 48 hours to 18 years old, who have return of circulation after cardiac arrest, who meet trial eligibility criteria, and whose guardians provide written consent.
Interventions: Therapeutic hypothermia or therapeutic normothermia.
Measurements and main results: From concept inception in 2002 until trial initiation in 2009, 7 years were required to plan and operationalize the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Two National Institute of Child Health and Human Development clinical trial planning grants (R21 and R34) supported feasibility assessment and protocol development. Two clinical research networks, Pediatric Emergency Care Applied Research Network and Collaborative Pediatric Critical Care Research Network, provided infrastructure resources. Two National Heart Lung Blood Institute U01 awards provided funding to conduct separate trials of in-hospital and out-of-hospital cardiac arrest. A pilot vanguard phase that included half the clinical sites began on March 9, 2009, and this was followed by full trial funding through 2015.
Conclusions: Over a decade will have been required to plan, design, operationalize, and conduct the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Details described in this report, such as participation of clinical research networks and clinical trial planning grants utilization, may be of utility for individuals who are planning investigator-initiated, federally supported clinical trials.
Trial registration: ClinicalTrials.gov NCT00878644 NCT00880087.
Figures

References
-
- The Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. - PubMed
-
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. - PubMed
-
- Shankaran S, Laptook AR, Ehrenkranz RA, et al. National Institute of Child Health and Human Development Neonatal Research Network: Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. - PubMed
-
- Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy Multicentre randomised trial. Lancet. 2005;365:663–670. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U01HD049934/HD/NICHD NIH HHS/United States
- R34 HD050531/HD/NICHD NIH HHS/United States
- U10HD049981/HD/NICHD NIH HHS/United States
- U03MC00003/PHS HHS/United States
- UG1 HD050096/HD/NICHD NIH HHS/United States
- U10 HD050096/HD/NICHD NIH HHS/United States
- U03MC00008/PHS HHS/United States
- HD044955/HD/NICHD NIH HHS/United States
- RL1 HD107773/HD/NICHD NIH HHS/United States
- U03MC00001/PHS HHS/United States
- HL094339/HL/NHLBI NIH HHS/United States
- U10HD050012/HD/NICHD NIH HHS/United States
- R21 HD044955/HD/NICHD NIH HHS/United States
- U10HD049945/HD/NICHD NIH HHS/United States
- U03MC00006/PHS HHS/United States
- U10HD049983,/HD/NICHD NIH HHS/United States
- U01 HL094339/HL/NHLBI NIH HHS/United States
- U10 HD050012/HD/NICHD NIH HHS/United States
- U10 HD049945/HD/NICHD NIH HHS/United States
- HL094345/HL/NHLBI NIH HHS/United States
- U10 HD049981/HD/NICHD NIH HHS/United States
- HD050531/HD/NICHD NIH HHS/United States
- U01 HL094345/HL/NHLBI NIH HHS/United States
- U10 HD049983/HD/NICHD NIH HHS/United States
- U03MC00007/PHS HHS/United States
- U10HD050096/HD/NICHD NIH HHS/United States
- U01 HD049934/HD/NICHD NIH HHS/United States
- U10HD500009/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

