Dual effect of heparin on Fe²⁺-induced cardiolipin peroxidation: implications for peroxidation of cytochrome c oxidase bound cardiolipin
- PMID: 23842788
- PMCID: PMC3783530
- DOI: 10.1007/s00775-013-1019-z
Dual effect of heparin on Fe²⁺-induced cardiolipin peroxidation: implications for peroxidation of cytochrome c oxidase bound cardiolipin
Abstract
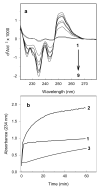
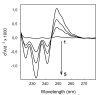
The effect of heparin on peroxidation of cardiolipin (CL) initiated by ferrous iron was studied in vitro using detergent-solubilized CL, liposomal CL, or CL bound to isolated cytochrome c oxidase (CcO). Heparin increased both the rate and the extent of CL peroxidation for detergent-solubilized CL and for CcO-bound CL. The effect of heparin was time- and concentration-dependent as monitored by the formation of conjugated dienes or thiobarbituric acid reactive substances. The results showed great similarity between the effect of heparin and the effect of certain iron chelators, such as ADP, on phospholipid peroxidation. Heparin increased the peroxidation of CcO-bound CL only when tertiary butyl hydroperoxide was also present. The enzyme activity of the resulting CcO complex decreased 25 %, in part due to peroxidation of functionally important CL. In contrast to peroxidation of detergent-solubilized CL, peroxidation of liposomal CL was inhibited by heparin, suggesting that the effect of heparin and ferrous iron depends on their proximity to the acyl chains of CL.
Figures




Similar articles
-
Bound cardiolipin is essential for cytochrome c oxidase proton translocation.Biochimie. 2014 Oct;105:159-64. doi: 10.1016/j.biochi.2014.07.005. Epub 2014 Jul 16. Biochimie. 2014. PMID: 25038566 Free PMC article.
-
Photolabeling of cardiolipin binding subunits within bovine heart cytochrome c oxidase.Biochemistry. 2006 Jan 24;45(3):746-54. doi: 10.1021/bi050870z. Biochemistry. 2006. PMID: 16411750 Free PMC article.
-
Cardiolipin-depleted bovine heart cytochrome c oxidase: binding stoichiometry and affinity for cardiolipin derivatives.Biochemistry. 1990 Sep 25;29(38):8962-9. doi: 10.1021/bi00490a012. Biochemistry. 1990. PMID: 2176838
-
Functional binding of cardiolipin to cytochrome c oxidase.J Bioenerg Biomembr. 1993 Apr;25(2):153-63. doi: 10.1007/BF00762857. J Bioenerg Biomembr. 1993. PMID: 8389748 Review.
-
The Interplay among Subunit Composition, Cardiolipin Content, and Aggregation State of Bovine Heart Cytochrome c Oxidase.Cells. 2020 Dec 3;9(12):2588. doi: 10.3390/cells9122588. Cells. 2020. PMID: 33287231 Free PMC article. Review.
Cited by
-
How hydrogen peroxide is metabolized by oxidized cytochrome c oxidase.Biochemistry. 2014 Jun 10;53(22):3564-75. doi: 10.1021/bi401078b. Epub 2014 May 30. Biochemistry. 2014. PMID: 24840065 Free PMC article.
-
Enoxaparin attenuates doxorubicin induced cardiotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis.BMC Pharmacol Toxicol. 2018 Jan 10;19(1):3. doi: 10.1186/s40360-017-0184-z. BMC Pharmacol Toxicol. 2018. PMID: 29321061 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

