UDP-GlcNAc 2-Epimerase/ManNAc Kinase (GNE): A Master Regulator of Sialic Acid Synthesis
- PMID: 23842869
- PMCID: PMC4161665
- DOI: 10.1007/128_2013_464
UDP-GlcNAc 2-Epimerase/ManNAc Kinase (GNE): A Master Regulator of Sialic Acid Synthesis
Abstract
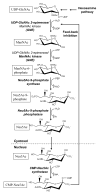
UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase is the key enzyme of sialic acid biosynthesis in vertebrates. It catalyzes the first two steps of the cytosolic formation of CMP-N-acetylneuraminic acid from UDP-N-acetylglucosamine. In this review we give an overview of structure, biochemistry, and genetics of the bifunctional enzyme and its complex regulation. Furthermore, we will focus on diseases related to UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase.
Figures

References
-
- Luchansky SJ, Yarema KJ, Takahashi S, Bertozzi CR. GlcNAc 2-epimerase can serve a catabolic role in sialic acid metabolism. J Biol Chem. 2003;278:8035–8042. - PubMed
-
- Hinderlich S, Berger M, Keppler OT, Pawlita M, Reutter W. Biosynthesis of N-acetylneuraminic acid in cells lacking UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase. Biol Chem. 2001;382:291–297. - PubMed
-
- Schauer R, Wember M. Isolation and characterization of sialate lyase from pig kidney. Biol Chem Hoppe Seyler. 1996;377:293–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

