Aryl C-H···Cl(-) hydrogen bonding in a fluorescent anion sensor
- PMID: 23843050
- PMCID: PMC4012615
- DOI: 10.1039/c3cc44574g
Aryl C-H···Cl(-) hydrogen bonding in a fluorescent anion sensor
Abstract
A new phenyl-acetylene receptor containing a carbonaceous hydrogen bond donor activates anion binding in conjunction with two stabilizing ureas. The unusual CH···Cl(-) hydrogen bond is apparent in solution by large (1)H NMR chemical shifts and by a short, linear contact in the solid state.
Figures
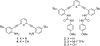



References
-
- Sessler JL, Gale PA, Cho W-S. Anion Receptor Chemistry. Royal Society of Chemistry; Cambridge: 2006.
-
- Beer PD, Gale PA. Angew. Chem. Int. Ed. 2001;40:486–516. - PubMed
-
-
Bordwell FG. Acc. Chem. Res. 1988;21:456–463. There appears to be discrepancy for the pKa of benzene in water, with values of 37 and 43 appearing most commonly. We chose to use the value reported in: Anslyn EV, Dougherty DA. Modern Physical Organic Chemistry. University Science Books; Sausalito, CA: 2006. p. 280.
-
-
- Desiraju GR, Steiner T. The Weak Hydrogen Bond: in Structural Chemistry and Biology. Oxford University Press; Oxford; New York: 1999.
-
- Schneider H-J. Angew. Chem. Int. Ed. 2009;48:3924–3977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

