Girard derivatization for LC-MS/MS profiling of endogenous ecdysteroids in Drosophila
- PMID: 23843360
- PMCID: PMC3708376
- DOI: 10.1194/jlr.D035949
Girard derivatization for LC-MS/MS profiling of endogenous ecdysteroids in Drosophila
Abstract
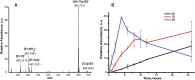
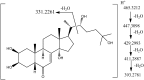
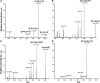
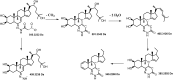
Ecdysteroids are potent developmental regulators that control molting, reproduction, and stress response in arthropods. In developing larvae, picogram quantities of individual ecdysteroids and their conjugated forms are present along with milligrams of structural and energy storage lipids. To enhance the specificity and sensitivity of ecdysteroid detection, we targeted the 6-ketone group, which is common to all ecdysteroids, with Girard reagents. Unlike other ketosteroids, during the reaction, Girard hydrazones of ecdysteroids eliminated the C14-hydroxyl group, creating an additional C14-C15 double bond. Dehydrated hydrazones of endogenous ecdysteroids were detected by LC-MS/MS in the multiple reaction monitoring (MRM) mode using two mass transitions: one relied upon neutral loss of a quaternary amine from the Girard T moiety; another complementary transition followed neutral loss of the hydrocarbon chain upon C20-C27 cleavage. We further demonstrated that a combination of Girard derivatization and LC-MS/MS enabled unequivocal detection of three major endogenous hormones at the picogram level in an extract from a single Drosophila pupa.
Keywords: Drosophila melanogaster; Girard reagent; ecdysone.
Figures
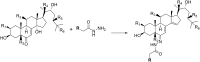


References
-
- Gilbert L. I. 2012. Insect Endocrinology. 1st edition. Elsevier/Academic Press, London; Waltham, MA
-
- Schwedes C. C., Carney G. E. 2012. Ecdysone signaling in adult Drosophila melanogaster. J. Insect Physiol. 58: 293–302 - PubMed
-
- Brogiolo W., Stocker H., Ikeya T., Rintelen F., Fernandez R., Hafen E. 2001. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 11: 213–221 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

