Behavioural fever is a synergic signal amplifying the innate immune response
- PMID: 23843398
- PMCID: PMC3730603
- DOI: 10.1098/rspb.2013.1381
Behavioural fever is a synergic signal amplifying the innate immune response
Abstract
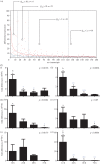
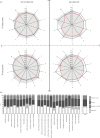
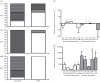
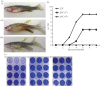
Behavioural fever, defined as an acute change in thermal preference driven by pathogen recognition, has been reported in a variety of invertebrates and ectothermic vertebrates. It has been suggested, but so far not confirmed, that such changes in thermal regime favour the immune response and thus promote survival. Here, we show that zebrafish display behavioural fever that acts to promote extensive and highly specific temperature-dependent changes in the brain transcriptome. The observed coupling of the immune response to fever acts at the gene-environment level to promote a robust, highly specific time-dependent anti-viral response that, under viral infection, increases survival. Fish that are not offered a choice of temperatures and that therefore cannot express behavioural fever show decreased survival under viral challenge. This phenomenon provides an underlying explanation for the varied functional responses observed during systemic fever. Given the effects of behavioural fever on survival and the fact that it exists across considerable phylogenetic space, such immunity-environment interactions are likely to be under strong positive selection.
Keywords: anti-viral response; behavioural fever; gene–environment interaction.
Figures

References
-
- Vaughn LK, Bernheim HA, Kluger MJ. 1974. Fever in the lizard Dipsosaurus dorsalis. Nature 252, 473–474 (doi:10.1038/252473a0) - DOI - PubMed
-
- Reynolds WW, Casterlin ME, Covert JB. 1976. Behavioural fever in teleost fishes. Nature 259, 41–42 (doi:10.1038/259041a0) - DOI - PubMed
-
- Casterlin ME, Reynolds WW. 1977. Behavioral fever in anuran amphibian larvae. Life Sci. 20, 593–596 (doi:10.1016/0024-3205(77)90461-1) - DOI - PubMed
-
- Casterlin ME, Reynolds WW. 1979. Fever induced in marine arthropods by prostaglandin E1. Life Sci. 25, 1601–1603 (doi:10.1016/0024-3205(79)90443-0) - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
